Analysis of oxidative stress-related markers in critically ill polytrauma patients: An observational prospective single-center study
DOI:
https://doi.org/10.17305/bjbms.2018.2306Keywords:
Polytrauma patients, trauma, vitamin C, N-acetylcysteine, antioxidants, oxidative stressAbstract
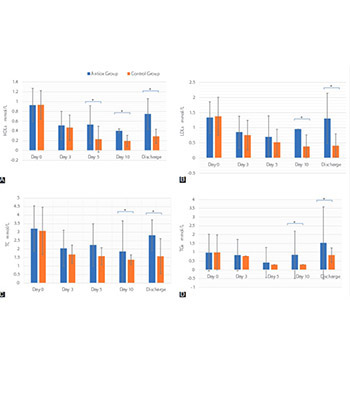
Critically ill polytrauma patients have increased production of free radicals (FRs) and consequent alterations in biochemical pathways, as well as disruption of cellular integrity, due to increased lipid peroxidation. The aim of this study was to investigate several biomarkers associated with increased oxidative stress in critically ill polytrauma patients, and to evaluate the effect of antioxidant treatment on the clinical outcome in these patients. A total of 67 polytrauma patients from an intensive care unit met the selection criteria. Antiox group included 35/67 patients who received antioxidant therapy, while 32/67 patients without antioxidant treatment were considered as control group. Antioxidant therapy consisted of simultaneous administration of Vitamin C (sodium ascorbate) and N-acetylcysteine, through continuous intravenous infusion. Clinical and paraclinical evaluation of the patients was performed daily until discharge or death. At admission, laboratory parameters did not differ significantly between two groups. At discharge/upon death, statistically significant differences in favor of Antiox group were observed in the following parameters: thrombocytes, activated partial thromboplastin time, prothrombin time, total bilirubin, total cholesterol, high-density lipoproteins, low-density lipoproteins, erythrocyte sedimentation rate, interleukin 6 (all p = 0.0001), total protein (p = 0.0005), serum albumin (p = 0.0004), lactate dehydrogenase (p = 0.0006), and C-reactive protein (p = 0.0014). Starting from day 5, the APACHE II score was significantly decreased in Antiox versus control group (p < 0.05). Finally, the sepsis incidence and mortality rate were significantly lower in Antiox group (p < 0.05). Decreasing the level of oxidative stress by antioxidant substances significantly correlated with a better prognosis and outcome in our patients. Further studies should elucidate more clearly the mechanism of action of antioxidants in critically ill polytrauma patients.
Citations
Downloads
References
Ciriello V, Gudipati S, Stavrou PZ, Kanakaris NK, Bellamy MC, Giannoudis PV. Biomarkers predicting sepsis in polytrauma patients: Current evidence. Injury 2013;44(12):1680-92. https://doi.org/10.1016/j.injury.2013.09.024.
Horton JW. Free radicals and lipid peroxidation mediated injury in burn trauma: The role of antioxidant therapy. Toxicology 2003;189(1-2):75-88.
https://doi.org/10.1016/S0300-483X(03)00154-9.
Khare M, Mohanty C, Das BK, Jyoti A, Mukhopadhyay B, Mishra SP. Free radicals and antioxidant status in protein energy malnutrition. Int J Pediatr 2014;2014:254396. https://doi.org/10.1155/2014/254396.
Rao PS, Sireesha K, Aparna Y, Sadanandam M. Free radicals and tissue damage: Role of antioxidants. Free Radicals Antioxidants 2011;1(4):2-7. https://doi.org/10.5530/ax.2011.4.2.
Espinosa-Diez C, Miguel V, Mennerich D, Kietzmann T, Sánchez-Pérez P, Cadenas S, et al. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol 2015;6:183-97. https://doi.org/10.1016/j.redox.2015.07.008.
Bedreag OH, Rogobete AF, Sarandan M, Cradigati AC, Papurica M, Rosu OM, et al. Influence of antioxidant therapy on the clinical status of multiple trauma patients. A retrospective single center study. Rom J Anaesth Intensive Care 2015;22(2):89-96.
Herford AS, Tandon R, Pivetti L, Cicciù M. Treatment of severe frontobasilar fractures in growing patients: A case series evaluation. Chin J Traumatol 2013;16(4):199-203. https://doi.org/10.3760/cma.j.issn.1008-1275.2013.04.002.
Cicciù M. Real opportunity for the present and a forward step for the future of bone tissue engineering. J Craniofac Surg 2017;28(3):592-3. https://doi.org/10.1097/SCS.0000000000003595.
Oudemans-van Straaten HM, Spoelstra-de Man AM, de Waard MC. Vitamin C revisited. Crit Care 2014;18(4):460. https://doi.org/10.1186/s13054-014-0460-x.
Kumar S, Sitasawad SL. N-acetylcysteine prevents glucose/glucose oxidase-induced oxidative stress, mitochondrial damage and apoptosis in H9c2 cells. Life Sci 2009;84(11-12):328-36. https://doi.org/10.1016/j.lfs.2008.12.016.
Cicciù M. A window view from the orient on trauma involving the inner maxillofacial region: From China to the global community with love. J Craniofac Surg 2016;27(1):6. https://doi.org/10.1097/SCS.0000000000002280.
Nogueira CR, Borges F, Lameu E, Franca C, Ramalho A. Effects of supplementation of antioxidant vitamins and lipid peroxidation in critically ill patients. Nutr Hosp 2013;28(5):1666-72. DOI: 10.3305/nh.2013.28.5.6590.
Shacter E, Williams JA, Lim M, Levine RL. Differential susceptibility of plasma proteins to oxidative modification: Examination by western blot immunoassay. Free Radic Biol Med 1994;17(5):429-37. https://doi.org/10.1016/0891-5849(94)90169-4.
Pawlak K, Borawski J, Naumnik B, Mysliwiec M. Relationship between oxidative stress and extrinsic coagulation pathway in haemodialyzed patients. Thromb Res 2003;109(5-6):247-51. https://doi.org/10.1016/S0049-3848(03)00241-X.
Dani C, Martelli E, Bertini G, Pezzati M, Filippi L, Rossetti M, et al. Plasma bilirubin level and oxidative stress in preterm infants. Arch Dis Child Fetal Neonatal Ed 2003;88(2):F119-23. https://doi.org/10.1136/fn.88.2.F119.
Brandes N, Schmitt S, Jakob U. Thiol-based redox switches in eukaryotic proteins. Antioxid Redox Signal 2009;11(5):997-1014. DOI: 10.1089/ars.2008.2285.
Rael LT, Bar-Or R, Aumann RM, Slone DS, Mains CW, Bar-Or D. Oxidation-reduction potential and paraoxonase-arylesterase activity in trauma patients. Biochem Biophys Res Commun 2007;361(2):561-5. https://doi.org/10.1016/j.bbrc.2007.07.078.
Gesquière L, Loreau N, Minnich A, Davignon J, Blache D. Oxidative stress leads to cholesterol accumulation in vascular smooth muscle cells. Free Radic Biol Med 1999;27(1-2):134-45. https://doi.org/10.1016/S0891-5849(99)00055-6.
Neher MD, Weckbach S, Flierl MA, Huber-Lang MS, Stahel PF. Molecular mechanisms of inflammation and tissue injury after major trauma-is complement the "bad guy"? J Biomed Sci 2011;18(1):90. DOI: 10.1186/1423-0127-18-90.
Reddell L, Cotton BA. Antioxidants and micronutrient supplementation in trauma patients. Curr Opin Clin Nutr Metab Care 2012;15(2):181-7. https://doi.org/10.1097/MCO.0b013e32835076df.
Nathens AB, Neff MJ, Jurkovich GJ, Klotz P, Farver K, Ruzinski JT, et al. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg 2002;236(6):814-22. https://doi.org/10.1097/00000658-200212000-00014.
Mahmood I, Tawfeek Z, El-Menyar A, Zarour A, Afifi I, Kumar S, et al. Outcome of concurrent occult hemothorax and pneumothorax in trauma patients who required assisted ventilation. Emerg Med Int 2015;2015:1-6. https://doi.org/10.1155/2015/859130.
Fama F, Cicciu M, Sindoni A, Nastro-Siniscalchi E, Falzea R, Cervino G, et al. Maxillofacial and concomitant serious injuries: An eight-year single center experience. Chin J Traumatol 2017;20(1):4-8. https://doi.org/10.1016/j.cjtee.2016.11.003.
Herford AS, Tandon R, Stevens TW, Stoffella E, Cicciu M. Immediate distraction osteogenesis: The sandwich technique in combination with rhBMP-2 for anterior maxillary and mandibular defects. J Craniofac Surg 2013;24(4):1383-7. https://doi.org/10.1097/SCS.0b013e318292c2ce.
Rogobete AF, Sandesc D, Papurica M, Stoicescu ER, Popovici SE, Bratu LM, et al. The influence of metabolic imbalances and oxidative stress on the outcome of critically ill polytrauma patients: A review. Burns Trauma 2017;5:8.
https://doi.org/10.1186/s41038-017-0073-0.
Horhat FG, Rogobete AF, Papurica M, Sandesc D, Tanasescu S, Dumitrascu V, et al. The use of lipid peroxidation expression as a biomarker for the molecular damage in the critically ill polytrauma patient. Clin Lab 2016;62(9):1601-7. https://doi.org/10.7754/Clin.Lab.2016.160306.
Bedreag OH, Rogobete AF, Sarandan M, Cradigati AC, Papurica M, Dumbuleu MC, et al. Oxidative stress in severe pulmonary trauma in critical ill patients. Antioxidant therapy in patients with multiple trauma-A review. Anaesthesiol Intensive Ther 2015;47(4):351-9. https://doi.org/10.5603/AIT.a2015.0030.
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2017-10-08
Published 2018-05-20









