Evaluation of bone mineral density (BMD) and indicators of bone turnover in patients with hemophilia
DOI:
https://doi.org/10.17305/bjbms.2018.2335Keywords:
Hemophilia, osteoporosis, bone mineral density, BMD, procollagen type I N-terminal propeptide, PINP, urinary N-terminal telopeptide, uNTX, complete blood count, CBC, bone resorption, bone formationAbstract
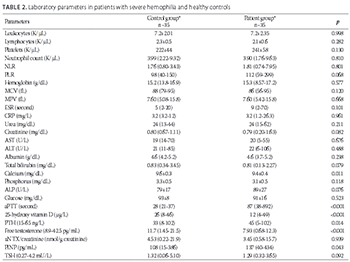
A decrease in bone mass is observed in hemophilic patients. The aim of this study was to evaluate bone mineral density (BMD), parathyroid hormone (PTH), 25-hydroxy vitamin D (vitamin D), and a bone formation and resorption marker, procollagen type I N-terminal propeptide (PINP) and urinary N-terminal telopeptide (uNTX) respectively, in hemophilic patients and healthy controls. Laboratory parameters related to the pathogenesis of bone loss such as neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) were also evaluated. Thirty-five men over 18 years of age, with severe hemophilia (A and B) and receiving secondary prophylaxis, were included in the study. The same number of age-, sex-, and ethnicity-matched healthy controls were evaluated. Anthropometric, biochemical, and hormonal parameters were determined in both groups. No significant difference in anthropometric parameters was found between the two groups. The BMD was low in 34% of hemophilic patients. Vitamin D, calcium, and free testosterone levels were significantly lower (p < 0.001, p = 0.011, p < 0.001, respectively), while PTH, PINP, and activated partial thromboplastin time (aPTT) levels were significantly higher (p < 0.014, p = 0.043, p < 0.001, respectively), in hemophilic patients compared to controls. There was no significant difference between the two groups in NLR, PLR, phosphorus, thyroid-stimulating hormone, and uNTX level. The reduction of bone mass in hemophilic patients may be evaluated using the markers of bone formation and resorption, enabling early detection and timely treatment.
Citations
Downloads
References
Stephensen D, Drechsler W, Scott O. Comparison of muscle strength and in-vivo muscle morphology in young children with haemophilia and those of age-matched peers. Haemophilia 2012;18(3):e302-10. https://doi.org/10.1111/j.1365-2516.2011.02705.x.
Hoyer LW. Haemophilia A. N Engl J Med 1994;330(1):38-47. https://doi.org/10.1056/NEJM199401063300108.
Anagnostis P, Vakalopoulou S, Slavakis A, Charizopoulou M, Kazantzidou E, Chrysopoulou T, et al. Reduced bone mineral density in patients with haemophilia A and B in Northern Greece. Thromb Haemost 2012;107(3):545-51.
https://doi.org/10.1160/TH11-08-05563.
Gerstner G, Damiano ML, Tom A, Worman C, Schultz W, Recht M, et al. Prevalence and risk factors associated with decreased bone mineral density in patients with haemophilia. Haemophilia 2009;15(2):559-65. https://doi.org/10.1111/j.1365-2516.2008.01963.x.
Katsarou O, Terpos E, Chatzismalis P, Provelengios S, Adraktas T, Hadjidakis D, et al. Increased bone resorption is implicated in the pathogenesis of bone loss in hemophiliacs: Correlations with hemophilic arthropathy and HIV infection. Ann Hematol 2010;89(1):67-74. https://doi.org/10.1007/s00277-009-0759-x.
Wallny TA, Scholz DT, Oldenburg J, Nicolay C, Ezziddin S, Pennekamp PH, et al. Osteoporosis in haemophilia – an underestimated comorbidity? Haemophilia 2007;13(1):79-84. https://doi.org/10.1111/j.1365-2516.2006.01405.x.
Linari S, Montorzi G, Bartolozzi D, Borderi M, Melchiorre D, Benelli M, et al. Hypovitaminosis D and and osteopenia/osteoporosis in a haemophilia population: A study in HCV/HIVor HCV infected patients. Haemophilia 2013;19(1):126-33.
https://doi.org/10.1111/j.1365-2516.2012.02899.x.
Kovacs CS. Haemophilia, low bone mass, and osteopenia/osteoporosis. Transfus Apheres Sci 2008;38(1):33-40. https://doi.org/10.1016/j.transci.2007.12.003.
Lewiecki EM, Watts NB, McClung MR, Petak SM, Bachrach LK, Shepherd JA, et al. Official positions of the International Society for Clinical Densitometry. J Clin Endocrinol Metab 2004;89(8):3651-5. https://doi.org/10.1210/jc.2004-0124.
Gurevitch O, Slavin S. The hematological etiology of osteoporosis. Med Hypotheses 2006;67(4):729-35. https://doi.org/10.1016/j.mehy.2006.03.051.
Kaufman JM, Reginster JY, Boonen S, Brandi ML, Cooper C, Dere W, et al. Treatment of osteoporosis in men. Bone 2013;53(1):134-44. https://doi.org/10.1016/j.bone.2012.11.018.
Brennan SL, Henry MJ, Wluka AE, Nicholson GC, Kotowicz MA, Pasco JA. Socioeconomic status and bone mineral density in a population-based sample of men. Bone 2010;46(4):993-9. https://doi.org/10.1016/j.bone.2009.12.029.
Watts NB, Adler RA, Bilezikian JP, Drake MT, Eastell R, Orwoll ES, et al. Osteoporosis in men: An endocrine society clinical practice guideline. J Clin Endocrinol Metab 2012;97(6):1802-22. https://doi.org/10.1210/jc.2011-3045.
Hoch AZ, Pajewski NM, Moraski L, Carrera GF, Wilson CR, Hoffmann RG, et al. Prevalence of the female athlete triad in high school athletes and sedentary students. Clin J Sport Med 2009;19(5):421-8. https://doi.org/10.1097/JSM.0b013e3181b8c136.
Anagnostis P, Vakalopoulou S, Vyzantiadis TA, Charizopoulou M, Karras S, Goulis DG, et al. The clinical utility of bone turnover markers in the evaluation of bone disease in patients with haemophilia A and B. Haemophilia 2014;20(2):268-75. https://doi.org/10.1111/hae.12271.
Alioglu B, Selver B, Ozsoy H, Koca G, Ozdemir M, Dallar Y. Evaluation of bone mineral density in Turkish children with severe haemophilia A: Ankara hospital experience. Haemophilia 2012;18(1):69-74. https://doi.org/10.1111/j.1365-2516.2011.02587.x.
Tlacuilo-Parra A, Villela-Rodríguez J, Garibaldi-Covarrubias R, Soto-Padilla J, Orozco-Alcala J. Bone turnover markers and bone mineral density in children with haemophilia. Haemophilia 2011;17(4):657-61. DOI: 10.1111/j.1365-2516.2010.02439.x.
Brown JP, Albert C, Nassar BA, Adachi JD, Cole D, Davison KS, et al. Bone turnover markers in the management of postmenopausal osteoporosis. Clin Biochem 2009;42(10-11):929-42. https://doi.org/10.1016/j.clinbiochem.2009.04.001.
Öztürk ZA, Yesil Y, Kuyumcu ME, Bilici M, Öztürk N, Yeşil NK, et al. Inverse relationship between neutrophil lymphocyte ratio (NLR) and bone mineral density (BMD) in elderly people. Arch Gerontol Geriatr 2013;57(1):81-5. https://doi.org/10.1016/j.archger.2013.02.005.
Huang C, Li S. Association of blood neutrophil lymphocyte ratio in the patients with postmenopausal osteoporosis. Pak J Med Sci 2016;32(3):762-5. https://doi.org/10.12669/pjms.323.10292.
Koseoglu SB. Bone loss & platelet-to-lymphocyte ratio. Biomark Med 2017;11(1):5-10. https://doi.org/10.2217/bmm-2016-0188.
Li XS, Zhang JR, Meng SY, Li Y, Wang RT. Mean platelet volume is negatively associated with bone mineral density in postmenopausal women. J Bone Miner Metab 2012;30(6):660-5. https://doi.org/10.1007/s00774-012-0362-4.
Hakala M, Kröger H, Valleala H, Hienonen-Kempas T, Lehtonen-Veromaa M, Heikkinen J, et al. Once-monthly oral ibandronate provides significant improvement in bone mineral density in postmenopausal women treated with glucocorticoids for inflammatory rheumatic diseases: A 12-month, randomized double-blind, placebo-controlled trial. Scand J Rheumatol 2012;41(4):260-6. https://doi.org/10.3109/03009742.2012.664647.
Azab B, Bhatt VR, Phookan J, Murukutla S, Kohn N, Terjanian T, et al. Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Ann Surg Oncol 2012;19(1):217-24.
https://doi.org/10.1245/s10434-011-1814-0.
Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, et al. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg 2008;32(8):1757-62.
https://doi.org/10.1007/s00268-008-9552-6.
Papa A, Emdin M, Passino C, Michelassi C, Battaglia D, Cocci F. Predictive value of elevated neutrophil-lymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin Chim Acta 2008;395(1-2):27-31. https://doi.org/10.1016/j.cca.2008.04.019.
Tamhane UU, Aneja S, Montgomery D, Rogers EK, Eagle KA, Gurm HS. Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol 2008;102(6):653-7. https://doi.org/10.1016/j.amjcard.2008.05.006.
Additional Files
Published
How to Cite
Accepted 2017-10-10
Published 2018-05-20









