In vitro fluoride release from a different kind of conventional and resin modified glass-ionomer cements
DOI:
https://doi.org/10.17305/bjbms.2013.2362Keywords:
fluoride release, glass-ionomers, resin modified glass-ionomers, cytotoxicityAbstract
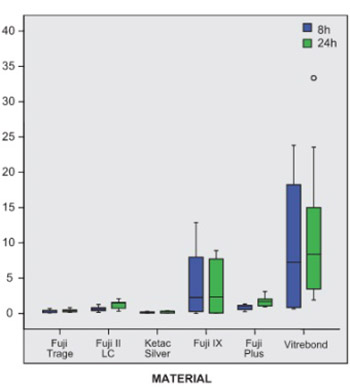
Fluoride release is important characteristic of glass-ionomer cements. Quantity of fluoride ions released from the glass-ionomer cements has major importance in definition of their biological activity. The objectives of this study were to define the quantity of fluoride ions released from the experimental glass-ionomer cements and to define the effect of fluoride ions released from the experimental glass-ionomer cements on their cytotoxicity. Concentrations of the fluoride ions released in the evaluated glass-ionomer cements were measured indirectly, by the fluoride-selective WTW, F500 electrode potential, combined with reference R503/D electrode. Statistical analyses of F-ion concentrations released by all glass-ionomers evaluated at two time points, after 8 and after 24 hours, show statistically higher fluoride releases from RMGICs: Vitrebond, Fuji II LC and Fuji Plus, when compared to conventional glass-ionomer cements: Fuji Triage, Fuji IX GP Fast and Ketac Silver, both after 8 and after 24 hours. Correlation coefficient between concentrations of fluoride ion released by evaluated glass-ionomer cements and cytotoxic response of UMR-106 osteoblast cell-line are relatively high, but do not reach levels of biological significance. Correlation between concentrations of fluoride ion released and cytotoxic response of NIH3T3 mouse fibroblast cell line after 8 hours is high, positive and statistically significant for conventional GICs, Fuji Triage and Fuji IX GP Fast, and RMGIC, Fuji II LC. Statistically significant Correlation coefficient between concentrations of fluoride ion released and cytotoxic response of NIH3T3 cell line after 24 hours is defined for RMGIC Fuji II LC only.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2017-08-13
Published 2013-08-20









