Simulation of left ventricular function during dyskinetic or akinetic aneurysm
DOI:
https://doi.org/10.17305/bjbms.2012.2442Keywords:
computer simulation, myocardial infarction, heart ventricle, aneurysmAbstract
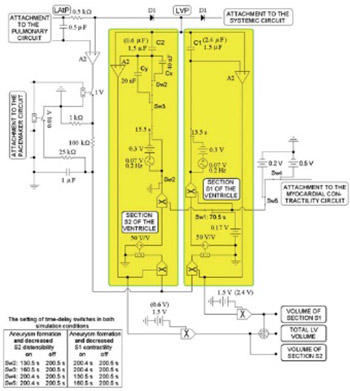
The purpose of our study was to simulate the hemodynamics of left ventricular function after left ventricular aneurysm (LVA) of various sizes and to validate the results of this computer based simulation with patient data. We developed an equivalent electronic circuit (EEC) that reflects the hemodynamic conditions of LVA (after acute myocardial infraction) while taking into consideration the resetting of the sympathetic nervous tone in the heart and systemic circuit, the fluctuating intrathoracic pressure during respiration and passive relaxation of the ventricle during diastole. The key feature of the EEC was a subcircuit representing the LVA, with a subcircuit to measure ventricular blood volume (i.e. intraventricular “shunting” of blood flow during systole and diastole) between the unaffected section of the left ventricle and its aneurysm. This EEC model can simulate akinetic or dyskinetic LVAs of different sizes and provides realistic beat-to-beat ventricular blood flow and pressure tracings that were validated by pressure-volume loop diagrams and by published patient data. In agreement with published data, simulated dyskinetic LVAs have a considerably greater impact on ventricular function than akinetic LVAs. The hemodynamic effects of ventricular systolic dysfunction following LVA were also evaluated. We conclude that the EEC model qualitatively and to a significant degree quantitatively represents conditions in patients with a dyskinetic or an akinetic LVA and provides realistic beat-to-beat ventricular blood flow and pressure tracings.
Citations
Downloads
Downloads
Additional Files
Published
How to Cite
Accepted 2017-09-13
Published 2012-11-20









