Prevalence of 1691G>A FV mutation in Poland compared with that in other Central, Eastern and South-Eastern European countries
DOI:
https://doi.org/10.17305/bjbms.2012.2500Keywords:
Factor V, FV Leiden, centroidsAbstract
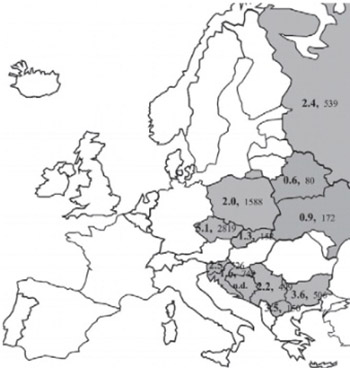
The 1691G>A FV variant has been described as a common genetic risk factor in venous thromboembolism. The purpose of this study was to provide a further frequency value for 1691G>A FV in Poland and to collate summary data from Central (Poland, Czech, Slovakia), Eastern (Russia, Belarus, Ukraine) and South-Eastern (Slovenia, Croatia, Bosnia and Herzegovina, Serbia, Montenegro, Macedonia, Bulgaria) European countries. For this purpose in 2007 the 1691G>A FV variant was analyzed by polymerase chain reaction-restriction fragment length polymorphism from DNA collected in 2005-2006. We studied 650 subjects: 400 newborns and 250 older individuals (mean age 46.1 y) from Poland and compared results with reports from other countries, as well as with the frequency trend of 845G>A HFE across South-Eastern European countries using centroid cities. From our 1691G>A FV study we identified 626 GG homozygotes, 23 GA heterozygotes, and i AA homozygote (n = 650), giving an A allele frequency of 1.9%, and a summed frequency value for Poland of 2.0% (n = 1588); the frequency in Central European countries was 3.9% (n = 4559), mostly due to the high value in the Czech Republic: 5.1% (n = 2819); the South-Eastern European countries had 2.5% (n = 2410). Among the Eastern European countries the 1691G>A FV allele frequency was 1.9% (n=791), between the South-Eastern and Eastern European countries there was no significant difference (p=0.17). We confirm that the 1691G>A FV allele frequency in Poland, as well as other countries compared, is significantly lower than that in Czech.
Citations
Downloads
Downloads
Additional Files
Published
How to Cite
Accepted 2017-09-26
Published 2012-05-20









