Trefoil factor family peptides TFF1 and TFF3 in the nervous tissues of developing mouse embryo
DOI:
https://doi.org/10.17305/bjbms.2015.251Keywords:
trefoil factor, nervous system, embryonic development, mouse, immunohistochemistryAbstract
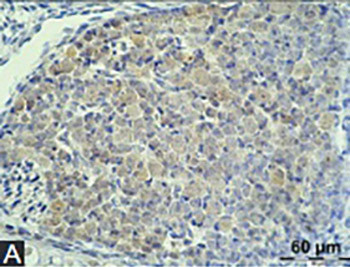
Trefoil factor family peptides (TFF1, TFF2, and TFF3) are predominantly found in mucous epithelia of various organs. However, they have also been reported in the nervous tissue, particularly mouse, rat, porcine, and human brain. The aim of this research was to determine the presence of TFF1 and TFF3 in the nervous system of developing mouse embryo. Mouse embryos, at the stages E15 to E17 were isolated, fixed in 4% paraformaldehyde and embedded in paraffin blocks. Sagittal 6µm sections were made, processed for immunohistochemistry, and incubated with anti-TFF1 or anti-TFF3 primary polyclonal rabbit antibodies. Labeled streptavidin-biotin method was used for TFF detection. TFF1 and 3 were found in the cytoplasm of ganglion cell somata, while TFF3 staining was also visible in the cytoplasm of neurons in different areas and nuclei of brain and medulla oblongata. Neurons in the gray matter of spinal cord were also TFF1 and TFF3 positive, and signal for both peptides was found in the choroid plexus. TFF peptides might be involved in the complex processes of nervous system development and differentiation and brain plasticity.
Citations
Downloads
References
Blin N. Cytoprotective trefoil peptides abound in new functions. Cell Mol Life Sci 2005;62:2907–9.
http://dx.doi.org/10.1007/s00018-005-5477-5
Hoffmann W, Jagla W, Wiede A. Molecular medicine of TFF-peptides: from gut to brain. Histol Histopathol 2001;16(1):319–34.
Madsen J, Nielsen O, Tornoe I, Thim L, Holmskov U. Tissue Localization of Human Trefoil Factors 1, 2, and 3. J Histochem Cytochem 2007;55:505–13.
http://dx.doi.org/10.1369/jhc.6A7100.2007
Regalo G, Wright NA, Machado JC. Trefoil factors: from ulceration to neoplasia. Cell Mol Life Sci 2005;62(24):2910–5.
http://dx.doi.org/10.1007/s00018-005-5478-4
Ahmed ARH, Griffiths AB, Tilby MT, Westley BR, May FEB. TFF3 is a normal breast epithelial protein and is associated with differentiated phenotype in early breast cancer but predisposes to invasion and metastasis in advanced disease. Am J Pathol 2012;180(3):904–16.
http://dx.doi.org/10.1016/j.ajpath.2011.11.022
Chaiyarit P, Utrawichian A, Leelayuwat C, Vatanasapt P, Chanchareonsook N, Samson MH, et al. Investigation of trefoil factor expression in saliva and oral mucosal tissues of patients with oral squamous cell carcinoma. Clin Oral Investig 2012;16(6):1549–56.
http://dx.doi.org/10.1007/s00784-011-0667-z
Dhar DK, Wang TC, Tabara H, Tonomoto Y, Maruyama R, Tachibana M, et al. Expression of trefoil factor family members correlates with patient prognosis and neoangiogenesis. Clin Cancer Res 2005;11(18):6472–8.
http://dx.doi.org/10.1158/1078-0432.CCR-05-0671
Kannan N, Kang J, Kong X, Tang J, Perry JK, Mohankumar KM, et al. Trefoil factor 3 is oncogenic and mediates anti-estrogen resistance in human mammary carcinoma. Neoplasia 2010;12(12):1041–53.
Kosriwong K, Menheniott TR, Giraud AS, Jearanaikoon P, Sripa B, Limpaiboon T. Trefoil factors: tumor progression markers and mitogens via EGFR/MAPK activation in cholangiocarcinoma. World J Gastroenterol 2011;17(12):1631–41.
http://dx.doi.org/10.3748/wjg.v17.i12.1631
Chaiyarit P, Chayasadom A, Wara-Aswapati N, Hormdee D, Sittisomwong S, Nakaresisoon S, et al. Trefoil factors in saliva and gingival tissues of patients with chronic periodontitis. J Periodontol 2012;83(9):1129–38.
http://dx.doi.org/10.1902/jop.2011.110431
Rösler S, Haase T, Claassen H, Schulze U, Schicht M, Riemann D, et al. Trefoil factor 3 is induced during degenerative and inflammatory joint disease, activates matrix metalloproteinases, and enhances apoptosis of articular cartilage chondrocytes. Arthritis Rheum 2010;62(3):815–25.
http://dx.doi.org/10.1002/art.27295
Wills-Karp M, Rani R, Dienger K, Lewkowich I, Fox JG, Perkins C, et al. Trefoil factor 2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection. J Exp Med 2012;209(3):607–22.
http://dx.doi.org/10.1084/jem.20110079
Wright NA, Poulsom R, Stamp G, Van Norden S, Sarraf C, Elia G, et al. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Scand J Gastroenterol Suppl 1992;193:76–82.
http://dx.doi.org/10.3109/00365529209096010
Baus-Loncar M, Kayademir T, Takaishi S, Wang T. Trefoil factor family 2 deficiency and immune response. Cell Mol Life Sci 2005;62(24):2947–55.
http://dx.doi.org/10.1007/s00018-005-5483-7
Hoffmann W. Trefoil factors TFF (trefoil factor family) peptide-triggered signals promoting mucosal restitution. Cell Mol Life Sci 2005;62(24):2932–8.
http://dx.doi.org/10.1007/s00018-005-5481-9
Thim L, Madsen F, Poulsen SS. Effect of trefoil factors on the viscoelastic properties of mucus gels. Eur J Clin Invest 2002;32(7):519–27.
http://dx.doi.org/10.1046/j.1365-2362.2002.01014.x
Hirota M, Awatsuji H, Sugihara Y, Miyashita S, Furukawa Y, Hayashi K. Expression of pS2 gene in rat brain. Biochem Mol Biol Int 1995;35(5):1079–84.
Hinz M, Schwegler H, Chwieralski CE, Laube G, Linke R, Pohle W, et al. Trefoil factor family (TFF) expression in the mouse brain and pituitary: changes in the developing cerebellum. Peptides 2004;25(5):827–32.
http://dx.doi.org/10.1016/j.peptides.2004.01.020
Hirota M, Awatsuji H, Furukawa Y, Hayashi K. Cytokine regulation of PS2 gene expression in mouse astrocytes. Biochem Mol Biol Int 1994;33(3):515–20.
Hoffmann W, Jagla W. Cell type specific expression of secretory TFF peptides: colocalization with mucins and synthesis in the brain. Int Rev Cytol 2002;213:147–81.
http://dx.doi.org/10.1016/S0074-7696(02)13014-2
Paunel-Görgülü AN, Franke AG, Paulsen FP, Dünker N. Trefoil factor family peptide 2 acts pro-proliferative and pro-apoptotic in the murine retina. Histochem Cell Biol 2011;135(5):461–73.
http://dx.doi.org/10.1007/s00418-011-0810-6
Jagla W, Wiede A, Dietzmann K, Rutkowski K, Hoffmann W. Co-localization of TFF3 peptide and oxytocin in the human hypothalamus. FASEB J 2000;14(9):1126–31.
Probst JC, Skutella T, Müller-Schmid A, Jirikowski GF, Hoffmann W. Molecular and cellular analysis of rP1.B in the rat hypothalamus: in situ hybridization and immunohistochemistry of a new P-domain neuropeptide. Brain Res Mol Brain Res 1995;33(2):269–76.
http://dx.doi.org/10.1016/0169-328X(95)00137-H
Schwarzberg H, Kalbacher H, Hoffmann W. Differential behavioral effects of TFF peptides: injections of synthetic TFF3 into the rat amygdala. Pharmacol Biochem Behav 1999;62(1):173–8.
http://dx.doi.org/10.1016/S0091-3057(98)00137-3
Schwarz H, Jagla W, Wiede A, Hoffmann W. Ultrastructural co-localization of TFF3-peptide and oxytocin in the neural lobe of the porcine pituitary. Cell Tissue Res 2001;305(3):411–6.
http://dx.doi.org/10.1007/s004410100412
Shi H-S, Yin X, Song L, Guo Q-J, Luo X-H. Neuropeptide Trefoil factor 3 improves learning and retention of novel object recognition memory in mice. Behav Brain Res 2012;227(1):265–9.
http://dx.doi.org/10.1016/j.bbr.2011.10.051
Paterson RW, Bartlett JW, Blennow K, Fox NC, Alzheimer's Disease Neuroimaging Initiative, Shaw LM, et al. Cerebrospinal fluid markers including trefoil factor 3 are associated with neurodegeneration in amyloid-positive individuals. Transl Psychiatry 2014;4(7):e419.
http://dx.doi.org/10.1038/tp.2014.58
Otto WR, Patel K. Trefoil factor family (TFF)-domain peptides in the mouse: embryonic gastrointestinal expression and wounding response. Anat Embryol (Berl) 1999;199(6):499–508.
http://dx.doi.org/10.1007/s004290050247
Gorba T, Bradoo P, Antonic A, Marvin K, Liu D-X, Lobie PE, et al. Neural regeneration protein is a novel chemoattractive and neuronal survival-promoting factor. Exp Cell Res 2006;312(16):3060–74.
http://dx.doi.org/10.1016/j.yexcr.2006.06.020
Bijelić N, Belovari T, Baus Lončar M. Trefoil factor family protein 3 (TFF3) is present in cartilage during endochondral ossification in the developing mouse fetus. Acta Histochem 2013;115(3):204–8.
http://dx.doi.org/10.1016/j.acthis.2012.06.007
Katusić A, Jurić-Lekić G, Jovanov-Milosević N, Vlahović M, Jezek D, Serman L, et al. Development of the fetal neural retina in vitro and in ectopic transplants in vivo. Coll Antropol 2008;32(1):201–7.
Downloads
Additional Files
Published
How to Cite
Accepted 2014-12-22
Published 2015-02-01









