Bronchioloalveolar differentiation in lung adenocarcinomas
DOI:
https://doi.org/10.17305/bjbms.2011.2560Keywords:
lung, adenocarcinoma, bronchioloalveolar carcinoma, alcian blue, immunohistochemistryAbstract
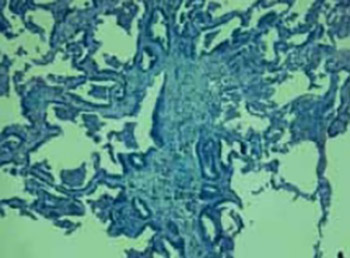
The goals of the study were to determine what percentage of neoplasms with a bronchioloalveolar (BAC) component were considered pure BAC by current World Health Organization (WHO) criteria. Next, we wanted to determine the number of mucinous BACs using histochemical staining with Alcian Blue PAS. Finally, we aimed to elucidate by immunohistochemistry the thyroid transcription factor-1 (TTF-1) frequency and cytokeratin 7 (CK7) expression, particularly in regard to the mucinous and non-mucinous subtypes of BAC tumors. We made a retrospective review of Hematoxylin and Eosin stained slides and classification of histologic grade, tumor subtype, and percentage of pure BAC pattern, with further characterization by histochemical staining for Alcian Blue PAS and Immunohistochemical staining for thyroid TTF-1 and CK7. Only 10 of 30 tumors examined could be classified as BAC by current strict WHO criteria. Nine cases were classified into non-mucinous and only one case was recognized as mucinous BAC, which showed positive staining for Alcian Blue PAS. TTF-1 positivity was in 100% of the non-mucinous BACs and complete absence of staining was in one case of mucinous BACs. CK7 expression in bronchioloalveolar carcinoma has demonstrated CK7 marked staining in 90% of non-mucinous BACs, also one case of mucinous BACs showed marked staining for TTF-1. BACs of mucinous morphology were notable for their conspicuous absence of TTF-1 immunoreactivity.
Citations
Downloads
Downloads
Additional Files
Published
How to Cite
Accepted 2017-10-14
Published 2011-11-20









