Radiographic estimation in seropositive and seronegative rheumatoid arthritis
DOI:
https://doi.org/10.17305/bjbms.2011.2571Keywords:
rheumatoid arthritis, seropositive, seronegative, radiography estimationAbstract
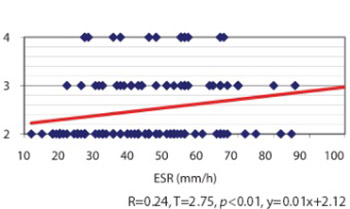
Long since it have been suggested that a subpopulation of patients with rheumatoid arthritis, diagnosed with negative rheumatoid factor tests, represents a clinical entity quite distinct from that of seropositive rheumatoid arthritis (RA). Our aim was to establish a scientific comparative analysis between seronegative and seropositive rheumatoid arthritis, regarding some radiological and clinical parameters, applied for the first time on patients from Kosovo. Two hundred fifty patients with rheumatoid arthritis according to the American College of Rheumatology criteria were retrospectively studied by analysis the radiographic damage and clinical parameters of the disease, using a data base. All examinees were between 25-60 years of age (Xb=49.96, SD=10.37) with disease duration between 1-27 years (Xb = 6.41, SD=6.47). All patients underwent a standardised evaluation radiographs. Baseline standardised poster anterior radiographs of hands and feet and radiographs of other joints, depending on indications, were assessed. Erythrocyte sedimentation rate values correlated with the radiological damages and statistical difference was found for seronegative subset (r=0.24, p<0.01). Longer duration of the disease resulted in the increase of radiological changes in both subsets (r=0.66, p<0.01) seronegative, (r=0.49, p<0.01) seropositive. Anatomic changes of IInd and IIIrd level were nearly equally distributed in both subsets, 76 (60.8%) seronegative, 75 (60%) seropositive. Radiological damages are nearly equal in both subsets, elevate in relation to the duration of the disease and correlate with ESR values. Regarding the sero-status, differences within sex, with some exceptions, are not relevant. Although there are some definite quantitative and qualitative differences regarding sero-status, obviously there is a great deal of overlap between the two groups.
Citations
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2017-10-17
Published 2011-08-20









