Celiac disease and fulminant T lymphoma detected too late in a 35-year-old female patient: Case report
DOI:
https://doi.org/10.17305/bjbms.2011.2573Keywords:
Celiac disease, EATL (Enteropathy-associated T cell lymphoma), RCD refractory celiac diseaseAbstract
Celiac disease is the most common chronic gastroenterological autoimmune disease characterized by gluten intolerance. The diagnosis of celiac disease and enteropathy-associated T cell lymphoma is often made when it is too late.
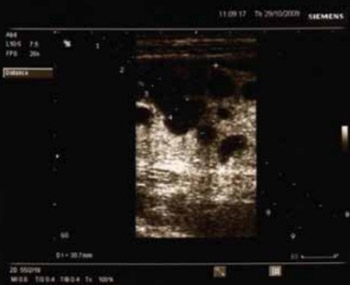
Case report describes a 35-year-old female patient managed for one year under the diagnosis of inflammatory bowel disease and admitted to our hospital for exacerbation of the underlying disease. However, inflammatory bowel disease was ruled out by diagnostic work-up, while the clinical picture and the findings obtained raised suspicion of lymphoma. The patient’s condition was additionally complicated by fulminant course of the disease and ileus.
Conclusion:Early diagnosis and appropriate treatment of the disease, and follow up of family members are crucial to prevent intestinal lymphoma development.
Citations
Downloads
Downloads
Additional Files
Published
How to Cite
Accepted 2017-10-17
Published 2011-08-20









