Skin and kidney histological changes in graft-versus-host disease (GVHD) after kidney transplantation
DOI:
https://doi.org/10.17305/bjbms.2011.2594Keywords:
experimental model, graft versus host disease, kidney transplantation skin biopsiesAbstract
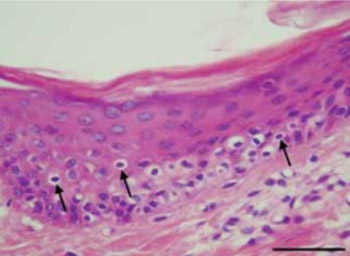
Kidney transplantation (Ktx) is generally performed during end stage renal disease due to a loss of the kidneys’ ability to filter wastes from the circulatory system. Acute graft-versus-host disease (GVHD) after Ktx is a life-threatening complication that progresses to organ failure, systemic complications, and death. The current study evaluated the significance of histologic findings of GVHD as obtained from skin biopsies following Ktx in swine. A swine model of Ktx with tacrolimus-based immunosuppression was used to assess possible correlations between acute-graft-cellular rejection and skin histological findings for prediction of GVHD. Animals were divided into a Ktx treatment group or a control group with no Ktx and skin and kidney biopsies were histologically assessed at postoperative days 0, 15, 30, 45 and 60. Skin samples were analyzed and classified from grade 1 to 4 of skin GVHD and the major histopathological changes of kidney acute cellular rejection were described using Banff’s score system. We observed a significant linear correlation between the histological grading values of skin biopsy changes and the histological grading values of kidney biopsies (Kendall’s tau_b=0.993) in the Ktx experimental group. No histological changes were observed in controls. Our findings demonstrate the diagnostic value of staging skin GVHD after Ktx and suggest it’s future utility for monitoring long term Ktx-induced changes.
Citations
Downloads
Additional Files
Published
How to Cite
Accepted 2017-10-22
Published 2011-05-20









