The tissue velocity imaging and strain rate imaging in the assessment of interatrial electromechanical conduction in patients with sick sinus syndrome before and after pacemaker implantation
DOI:
https://doi.org/10.17305/bjbms.2011.2595Keywords:
sick sinus syndrome, atrial pacing, electromechanical conduction, tissue velocity imaging, strain rate imagingAbstract
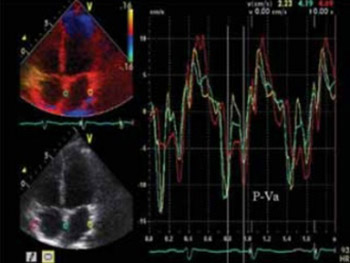
Tissue velocity imaging (TVI) and strain rate imaging (SRI) were recently introduced to quantify myocardial mechanical activity in patients receiving cardiac resynchronization therapy. To clear whether atrial-demand-based (AAI) (R) atrial pacing can fully simulate the electromechanical conduction of physiological state and to clarify which one is more appropriate for the assessment of electromechanical activity of the heart between TVI and SRI, 30 normal subjects and 31 patients with sick sinus syndrome (SSS) before and after AAI(R) pacemaker implantation (PI) were investigated in this study. The results showed that the time intervals (ms), P-SRa assessed by SRI (not P-Va assessed by TVI) prolonged step by step from the lateral wall of the right atrium (RA), the interatrial septum (IAS) and the left atrium (LA) in normal subjects(5.01±0.62, 17.05±3.54 and 45.09±12.26, p<0.01). P-Va and P-SRa did not differ at the RA, IAS and LA in patients with SSS before PI (p>0.05), and they were significant longer than those of normal subjects (p<0.01). However, they shortened to normal levels in patients with SSS after PI and P-SRa showed again the trend of gradually prolonging from the RA, IAS to LA. At the same time, the peak velocities and the peak strain rates during atrial contraction also returned to normal values from lower levels. These data suggested that AAI(R) atrial pacing can successfully reverse the abnormal interatrial electromechanical conduction in patients with SSS, and SRI is more appropriate for the assessment of the electromechanical activity of atrial wall than TVI.
Citations
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2017-10-23
Published 2011-05-20









