Hypoglycaemic action of stevioside and a barley and brewer’s yeast based preparation in the experimental model on mice
DOI:
https://doi.org/10.17305/bjbms.2011.2616Keywords:
stevioside, barley and brewer’s yeast extract, alloxan, diabetes mellitusAbstract
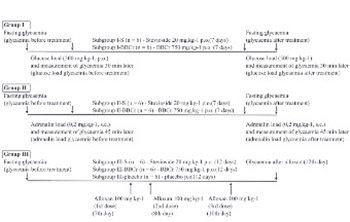
The aim of this study was to investigate influence of the preparation based on barley and brewer’s yeast extracts with chromium (BBCr) and stevioside (S) on fasting glycaemia and glycaemia in mice after glucose, adrenalin and alloxan application. The animals were divided into three groups: glucose 500 mgkg-1 (I); adrenalin 0.2 mgkg-1(II) and alloxan 100 mg kg-1 (III) and into subgroups according to the substance they received: stevioside 20 mg kg-1 (I-S, II-S, III-S); BBCr 750 mg kg-1(I-BBCr, II-BBCr, III-BBCr) and saline 1ml/100g (III-placebo). Glycaemia was measured before and after 7-day treatment with stevioside or BBCr in the following conditions: fasting, 30min after glucose load (I) or 45min after adrenaline load (II). In group III glycaemia was measured before and after 12-day treatment with S, BBCr or placebo and alloxan application (7th, 8th and 10th days of treatment ). BBCr significantly reduced fasting glycaemia in I and II groups and glycaemia values after the glucose load (I-BBCr: 9.20 ± 0.61 vs. 7.42 ± 0.59 mmol/L, p = 0.01). Stevioside significantly reduced glycaemia after the adrenalin load (II-S: 13.45 ± 0.71 vs. 11.65 ± 1.19 mmol/L; p = 0.03). In the III-BBCr glycaemia values did not indicate the development of alloxan-induced diabetes and were significantly lower than in the III-placebo (8.6 ± 3.16 vs. 18.8 ± 5.53 mmol/L; p < 0.05). In conclusion, BBCr caused a significant decrease of fasting glycaemia, significant reduction of glycaemia after glucose load and prevented onset of alloxan-induced diabetes. Stevioside caused the decrease of adrenalin-induced hyperglycaemia.
Citations
Downloads
Additional Files
Published
How to Cite
Accepted 2017-11-01
Published 2011-02-20









