Plasma nitric oxide and left ventricular function in rabbits after cardiac lymphatic obstruction
DOI:
https://doi.org/10.17305/bjbms.2011.2617Keywords:
cardiac lymphatic vessels, endothelium, nitric oxide, left ventricular function, rabbitsAbstract
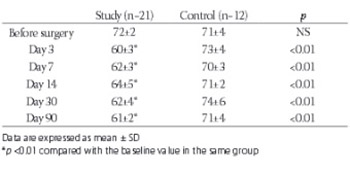
This study was designed to investigate the effect of cardiac lymphatic obstruction on plasma nitric oxide (NO) and left ventricular function. The plasma NO was measured in study group (n=21) and control group rabbits (n=12) before, and 3, 7, 14, 30 and 90 days after the obstruction of cardiac lymphatic vessels. Left ventricular ejection fraction was measured with echocardiography. There was a significant reduction in the left ventricular ejection fraction following the lymphatic obstruction (0.72±0.02 vs. 0.61±0.02, p<0.01). Plasma NO in the control group remained unchanged during the observation period (54.2±4.4 vs. 52.0±4.2 μmol/L, p>0.05). In the study group, there was a small but significant increase in the plasma NO on day 3, 7 and 14 following the lymphatic obstruction (52.3±4.1 vs. 73.4±5.9 μmol/L, p<0.01). The plasma NO returned to the baseline levels on day 30 but reduced to 44.9±3.6 pmol/L on 90 days after the lymphatic obstruction (p<0.05). In conclusion, cardiac lymphatic obstruction was associated with a significant reduction in left ventricular function. It was also associated with an increase in the plasma NO in the first 2 weeks but there was a significant reduction in the NO levels three months after the lymphatic obstruction.
Citations
Downloads
Additional Files
Published
How to Cite
Accepted 2017-11-01
Published 2011-02-20









