Cerebral aneurysm associated with cardiac myxoma: Case Report
DOI:
https://doi.org/10.17305/bjbms.2011.2629Keywords:
atrial myxoma, left atrium, cerebral aneurysmAbstract
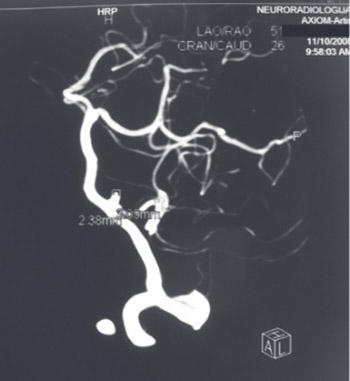
Left atrial myxomas are a rare but well known cause of cerebrovascular accidents in young people. Cerebral embolism is the most common cause of cerebral ischemic stroke. The intracranial aneurysm is rarely associated with myxoma. We report the case of a patient who had an operation of PICA aneurysm due to subarachnoid hemorrhage ten months before the discovery of the large left atrial myxoma. Fortunately, the untimely diagnosis of the myxoma did not have other consequences. In order to prevent possible complications of we should keep in mind that these two apparently different entities could be associated.
Citations
Downloads
Additional Files
Published
How to Cite
Accepted 2017-11-02
Published 2011-02-20









