Interleukin-4 (IL4) -590C/T (rs2243250) gene polymorphism is not associated with diabetic nephropathy (DN) in Caucasians with type 2 diabetes mellitus (T2DM)
DOI:
https://doi.org/10.17305/bjbms.2018.2688Keywords:
Interleukin 4, rs2243250, diabetic nephropathy, association studyAbstract
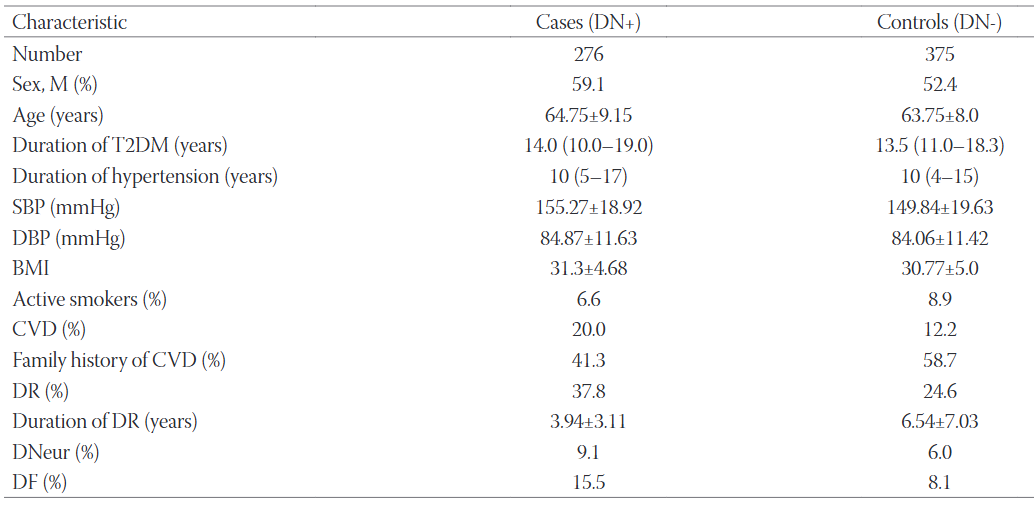
Diabetic nephropathy (DN) is a microvascular complication that affects up to 40% of diabetic patients and can lead to end-stage kidney disease. Inflammatory cytokines such as interleukin 1 (IL-1), IL-6, IL-18 and tumor necrosis factor-α (TNFα) have been linked to the development and progression of DN. The aim of our study was to examine the relationship between interleukin-4 (IL4) -590C/T (rs2243250) gene polymorphism and DN in patients with type 2 diabetes mellitus (T2DM). This study is a continuation of our previous research on the association between angiotensinogen (AGT) gene polymorphisms and DN in patients with T2DM. We included 651 unrelated Slovenian (Caucasian) patients who had had T2DM for at least 10 years. The participants were classified into a group of T2DM patients with DN (276 cases) and a group without DN (375 controls). IL4 rs2243250 polymorphism was analyzed using a TaqMan SNP genotyping assay and StepOne Real-Time PCR System. The frequencies of rs2243250 TT, CT and CC (wild type) genotypes were 3.2%, 29.4% and 67.4%, respectively in patients with DN, and 2.7%, 34.4% and 62.9%, respectively in controls. Our logistic regression analysis adjusted for gender, age, diabetes duration, and glycated hemoglobin showed no association between rs2243250 and the risk for DN (OR 1.06; CI 0.37-3.05; p = 0.9). IL4 rs2243250 is not associated with DN in our subset of Slovenian patients with T2DM.
Citations
Downloads
References
Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, et al. IDF diabetes atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract 2017;128:40-50. https://doi.org/10.1016/j.diabres.2017.03.024.
Boyle JP, Honeycutt AA, Narayan KM, Hoerger TJ, Geiss LS, Chen H, et al. Projection of diabetes burden through 2050: Impact of changing demography and disease prevalence in the U.S. Diabetes Care 2001;24(11):1936-40. https://doi.org/10.2337/diacare.24.11.1936.
Ayodele OE, Alebiosu CO, Salako BL. Diabetic nephropathy - A review of the natural history, burden, risk factors and treatment. J Natl Med Assoc 2004;96(11):1445-54.
Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, et al. Kidney disease and increased mortality risk in type 2 diabetes. J Am Soc Nephrol 2013;24(2):302-8. https://doi.org/10.1681/ASN.2012070718.
Freedman BI, Bostrom M, Daeihagh P, Bowden DW. Genetic factors in diabetic nephropathy. Clin J Am Soc Nephrol 2007;2(6):1306-16. https://doi.org/10.2215/CJN.02560607.
Navarro-González JF, Mora-Fernández C. The role of inflammatory cytokines in diabetic nephropathy. J Am Soc Nephrol 2008;19(3):433-42. https://doi.org/10.1681/ASN.2007091048.
Paul WE. Interleukin-4: A prototypic immunoregulatory lymphokine. Blood 1991;77(9):1859-70.
Vitetta ES, Ohara J, Myers CD, Layton JE, Krammer PH, Paul WE. Serological, biochemical, and functional identity of B cell-stimulatory factor 1 and B cell differentiation factor for IgG1. J Exp Med 1985;162(5):1726-31. https://doi.org/10.1084/jem.162.5.1726.
Mosmann TR, Coffman RL. Th1 and Th2 cells: Different pattern of lymphokine secretion lead to different functional properties. Annu Rev Immunol 1898;7:145-73. https://doi.org/10.1146/annurev.iy.07.040189.001045.
Seder RA, Paul WE, Davis MM, Fazekas de St Groth B. The presence of interleukin 4 during in vitro priming determines the lymphokine-producing potential of CD4+ T cells from T cell receptor transgenic mice. J Exp Med 1992;176(4):1091-8. https://doi.org/10.1084/jem.176.4.1091.
Zamorano J, Rivas MD, Pérez-GM. Interleukin-4: A multifunctional cytokine. Inmunología 2003;22(2):215-24.
Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol 1996;14:397-440. https://doi.org/10.1146/annurev.immunol.14.1.397.
Steinke JW, Borish L. Th2 cytokines and asthma-interleukin-4: Its role in the pathogenesis of asthma, and targeting it for asthma treatment with interleukin-4 receptor antagonists. Respir Res 2001;2(2):66-70. https://doi.org/10.1186/rr40.
Mi QS, Ly D, Zucker P, McGarry M, Delovitch TL. Interleukin-4 but not interleukin-10 protects against spontaneous and recurrent Type 1 diabetes by activated CD1d-restricted invariant natural killer T-cells. Diabetes 2004;53(5):1303-10. https://doi.org/10.2337/diabetes.53.5.1303.
Mire-Sluis AR. Interleukin-4. In: Cytokines. London: Academic Press; 1998. p. 53-68. https://doi.org/10.1016/B978-012498340-3/50005-1.
Liang J, Liu Y, Xue R, Chen L, Chen H, Shao L, et al. Interleukin 4 -590C/T (rs2243250) polymorphism is associated with increased risk of atopic dermatitis: Meta-analysis of case-control studies. Dermatitis 2017;28(2):144-51. https://doi.org/10.1097/DER.0000000000000265.
Qiu LJ, Ni J, Cen H, Wen PF, Zhang M, Liang Y, et al. Relationship between the IL-4 gene promoter -590C/T (rs2243250) polymorphism and susceptibility to autoimmune diseases: A meta-analysis. J Eur Acad Dermatol Venereol 2015;29(1):48-55. https://doi.org/10.1111/jdv.12435.
Liu S, Li T, Liu J. Interleukin-4 rs2243250 polymorphism is associated with asthma among Caucasians and related to atopic asthma. Cytokine 2012;59(2):364-9. https://doi.org/10.1016/j.cyto.2012.05.006.
Makuc J, Šeruga M, Završnik M, Cilenšek I, Petrovič D. Angiotensinogen (AGT) gene missense polymorphisms (rs699 and rs4762) and diabetic nephropathy in caucasians with Type 2 diabetes mellitus. Bosn J Basic Med Sci 2017;17(3):262-7. https://doi.org/10.17305/bjbms.2017.1823.
Zheng Z, Zheng F. Immune cells and inflammation in diabetic nephropathy. J Diabetes Res 2016;2016:1841690. https://doi.org/10.1155/2016/1841690.
Kazemi Arababadi M. Interleukin-4 gene polymorphisms in Type 2 diabetic patients with nephropathy. Iran J Kidney Dis 2010;4(4):302-6.
Neelofar K, Ahmad J, Ahmad A, Alam K. Study of IL4-590C/T and IL6-174G/C gene polymorphisms in type 2 diabetic patients with chronic kidney disease in North Indian population. J Cell Biochem 2017;118(7):1803-9. https://doi.org/10.1002/jcb.25853.
Alsaid A, El-Missiry M, Hatata ES, Tarabay M, Settin A. Association of IL-4-590 C>T and IL-13-1112 C>T gene polymorphisms with the susceptibility to Type 2 diabetes mellitus. Dis Markers 2013;35(4):243-7. https://doi.org/10.1155/2013/107470.
Cilenšek I, Hercegovac A, Nikolajević Starčević J, Vukojević K, Saraga Babić M, Milutinović Živin A. Polymorphisms of interleukin-4, -10 and 12B genes and diabetic retinopathy. Central Eur J Biol 2011;6(4):558-64. https://doi.org/10.2478/s11535-011-0036-6.
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2018 Bosnian Journal of Basic Medical Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2018-01-28
Published 2018-11-07









