Downregulation of TSPAN13 by miR-369-3p inhibits cell proliferation in papillary thyroid cancer (PTC)
DOI:
https://doi.org/10.17305/bjbms.2018.2865Keywords:
Papillary thyroid cancer, PTC, miR-369-3p, TSPAN13, tetraspanin-13, cell proliferation, microRNAAbstract
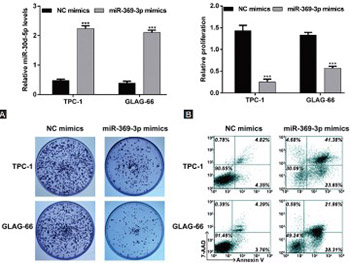
Previous studies demonstrated dysregulation of different microRNAs in thyroid cancer. Tetraspanins (TSPANs) are cell surface proteins with critical roles in many cellular processes, and implications in tumor development. Here we investigated the role of miR-369-3p in papillary thyroid cancer (PTC) and its association with TSPAN13. miR-369-3p and the TSPAN13 gene expression profiles of 513 thyroid cancer and 59 normal thyroid tissues were downloaded from the Cancer Genome Atlas database. Thyroid cancer tissues were classified according to the histological type, grouped based on low and high median miR-369-3p and TSPAN13 expression, and analyzed in relation to overall survival (OS) of patients. Human PTC cell lines (TPC-1 and GLAG-66) and human embryonic kidney 293T (HEK293T) cells were used for in vitro analysis. Transfection experiments were performed with synthetic miRNA mimics for miR-369-3p and small interfering RNAs for TSPAN13. Relative expression of miR-369-3p and TSPAN13 mRNA was determined by RT-qPCR. Protein levels of TSPAN13 were determined by western blotting. Cell proliferation (CCK-8 assay), colony formation, and apoptosis (flow cytometry) were analyzed in transfected cells. Binding sites of miR-369-3p in TSPAN13 mRNA were determined by bioinformatics analysis and dual luciferase reporter assay. miR-369-3p was downregulated and TSPAN13 upregulated in PTC, follicular thyroid cancer, and tall cell variant tissues. Both low expression of miR-369-3p and high expression of TSPAN13 were associated with shorter OS in thyroid cancer patients. Overexpression of miR-369-3p significantly suppressed proliferation and promoted apoptosis in PTC cells. TSPAN13 was a direct target of miR-369-3p, and silencing of TSPAN13 phenocopied the effect of miR-369-3p mimics in PTC cells. Overall, the downregulation of miR-369-3p and consequent upregulation of its target TSPAN13 appear to be involved in pathophysiology of PTC.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2018-01-30
Published 2019-05-20









