Immunization with 3-oxododecanoyl-L-homoserine lactone-r-PcrV conjugate enhances survival of mice against lethal burn infections caused by Pseudomonas aeruginosa
DOI:
https://doi.org/10.17305/bjbms.2015.292Keywords:
3-oxo-C12-HSL, Homoserine lactone (HSL), Immunization, PcrV, Pseudomonas aeruginosa, VaccineAbstract
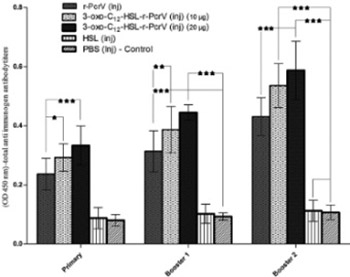
Quorum Sensing and type III secretion system play an important role in the virulence of Pseudomonas (P.) aeruginosa in burn wound infections. We aimed to explore the feasibility of using 3-oxo-C12-HSL-r-PcrV conjugate as a candidate vaccine against P. aeruginosa caused infections. 3-oxo-C12-HSL-r-PcrV conjugate was prepared and used for immunization of mice (10 μg, subcutaneous, three times, at 2-week intervals). Mice were divided into five groups: I: PcrV; II: 3-oxo-C12-HSL-r-PcrV (10 μg); III: 3-oxo-C12-HSL-r-PcrV (20 μg); IV: 3-oxo-C12-HSL; and V: PBS receiving groups. After each shot of immunization, total and isotype antibody responses against corresponding antigen were measured to determine the immunization efficacy. One month after the last immunization, all groups were burned and challenged subeschar with P. aeruginosa PAO1. Survival rate and bacterial quantity in the skin and internal organs (liver and spleen) were evaluated 25-hr after burn infection. Immunization with 3-oxo-C12-HSL-r-PcrV significantly increased total IgG and specific subclass antibodies (IgG1, IgG2a, IgG2b, and IgM) in the serum of the groups II and III compared to the control group (p<0.001). While all the control mice (PBS injected group) died within 2 days after bacterial challenge, 64% of the group I, 78% of group II, and 86% of group III, survived within 14 days after challenge. Interestingly, bacterial burden in the liver and spleen of 3-oxo-C12-HSL-r-PcrV injected group (III) was significantly lower than the control group (p<0.001). The present study proposed two-component vaccine to inhibit Pseudomonas infections in burned mouse.
Citations
Downloads
References
: Richards M J, Edwards J R, Culver D H, Gaynes R P. Nosocomial infections in medical intensive care units in the United States. National Nosocomial Infections Surveillance System. Crit Care Med 1999;27:887–892.
http://dx.doi.org/10.1097/00003246-199905000-00020
:Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clinical microbiology reviews 2006;19(2):403-34.
http://dx.doi.org/10.1128/CMR.19.2.403-434.2006
:Erol S, Altoparlak U, Akcay MN, Celebi F, Parlak M. Changes of microbial flora and wound colonization in burned patients. Burns 2004;30(4):357-61.
http://dx.doi.org/10.1016/j.burns.2003.12.013
: Poole K, Srikumar R. Multidrug efflux in Pseudomonas aeruginosa: components, mechanisms and clinical significance. Curr Top Med Chem 2001;1:59-71.
http://dx.doi.org/10.2174/1568026013395605
: Schweizer HP. Efflux as a mechanism of resistance to antimicrobials in Pseudomonas aeruginosa and related bacteria: unanswered questions. Genet Mol Res. 2003;2(1):48-62.
: Erol S, Altoparlak U, Akcay M N, Celebi F, Parlak M. Changes of mi¬crobial flora and wound colonization in burned patients. Burns. 2004; 30(4):357-61.
http://dx.doi.org/10.1016/j.burns.2003.12.013
: Fuqua WC, Winans SC, Greenberg EP. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J of bacteriol. 1994;176(2):269.
: Van Delden C, Iglewski B H. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis 1998;4:551–560.
http://dx.doi.org/10.3201/eid0404.980405
: Pearson J P, Gray K M, Passador L, Tucker K D, Eberhard A, et al. Structure of the autoinducer required for expression of Pseudomonas aeruginosa virulence genes. Proc Natl Acad Sci USA 1994; 91:197–201.
http://dx.doi.org/10.1073/pnas.91.1.197
: Pearson JP, Passador L, Iglewski BH, Greenberg E. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. PNAS. 1995;92(5):1490-4.
: Miller M B, Bassler B L. Quorum sensing in bacteria. Annu Rev Microbiol 2001;(55):165–199.
http://dx.doi.org/10.1146/annurev.micro.55.1.165
: Smith RS, Iglewski BH. Pseudomonas aeruginosa quorum sensing as a potential antimicrobial target. J Clin Invest 2003;(112):1460–1465.
http://dx.doi.org/10.1172/JCI200320364
: Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol Rev 2001;(25):365–404.
http://dx.doi.org/10.1111/j.1574-6976.2001.tb00583.x
:Tateda K, Ishii Y, Horikawa M, Matsumoto T, Miyairi S, Pechere J C., et al. Pseudomonas aeruginosa autoinducer N-3-oxododecanoylhomoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect Immun 2003;(71):5785–5793.
http://dx.doi.org/10.1128/IAI.71.10.5785-5793.2003
: DiMango E, Zar H J, Bryan R, Prince A. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J Clin Invest 1995;(96)2204–2210.
http://dx.doi.org/10.1172/JCI118275
: Saleh A, Figarella C, Kammouni W, Marchand-Pinatel S, Lazdunski A, Tubul A, et al. Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-L-homoserine lactone inhibits expression of P2Y receptors in cystic fibrosis tracheal gland cells. Infect. Immun. 1999;67(10):5076-82.
: Telford G, Wheeler D, Williams P, Tomkins P, Appleby P, Sewell H, et al. The Pseudomonas aeruginosaQuorum-Sensing Signal MoleculeN-(3-Oxododecanoyl)-l-Homoserine Lactone Has Immunomodulatory Activity. Infect. Immun. 1998;66(1):36-42.
: Suga H, Smith K M. Molecular mechanisms of bacterial quorum sensing as a new drug target. Curr Opin Chem Biol 2003;(7)586–591.
http://dx.doi.org/10.1016/j.cbpa.2003.08.001
: Smith RS, R Kelly, Iglewski BH, Phipps RP. The Pseudomonas autoinducer N-(3-oxododecanoyl) homoserine lactone induces cyclo-oxygenase-2 and prostaglandin E2 production in human lung fibroblasts: Implications for inflammation. J Immunol 2002;(169):2636–2642.
http://dx.doi.org/10.4049/jimmunol.169.5.2636
: Rumbaugh KP, Griswold JA, Hamood AN. The role of quorum sensing in the in vivo virulence of Pseudomonas aeruginosa. Microbes Infect 2000(2):1721-1731.
http://dx.doi.org/10.1016/S1286-4579(00)01327-7
: Sperandio V, Torres AG, Jarvis B, Nataro JP, Kaper JB. Bacteria–host communication: the language of hormones. PNAS. 2003;100(15):8951-6.
: Barman A, Savel R H, S. Racine,et al. Type III protein secretion is associated with death in lower respiratory and systemic Pseudomonas aeruginosa infections. J.Infect. Dis. 2001; (183):1767-1774.
http://dx.doi.org/10.1086/320737
: Vallis AJ, Yahr TL, Barbieri JT, Frank DW. Regulation of ExoS Production and Secretion by Pseudomonas aeruginosa in Response to Tissue Culture Conditions. Infect. Immun. 1999;67(2):914-20.
: Imamura Y, Yanagihara K, Fukuda Y, Kaneko Y, Seki M, Izumikawa K, et al. Effect of anti-PcrV antibody in a murine chronic airway P.aeruginosa infection model. European Respiratory Journal 2007;(5):965-8.
http://dx.doi.org/10.1183/09031936.00147406
: Holder IA, Neely AN, Frank DW. PcrV immunization enhances survival of burned P. aeruginosa -infected mice. Infect. Immun.2001;(9):5908-10.
http://dx.doi.org/10.1128/IAI.69.9.5908-5910.2001
: Sawa T, Yahr TL, Ohara M, Kurahashi K, Gropper MA, Wiener-Kronish JP, et al. Active and passive immunization with the Pseudomonas V antigen protects against type III intoxication and lung injury. Nat. Med. 1999;5(4):392-8.
: Miyairi S,Tateda K. Immunization with 3-oxododecanoyl-L-homoserine lactone–protein conjugate protects mice from lethal Pseudomonas aeruginosa lung infection. J.Med.Microbiol 2006;(55):1381–1387
http://dx.doi.org/10.1099/jmm.0.46658-0
: Kyd JM, Cooley M. Conjugates of acyl homoserine lactone and catalase a from pseudomonas aeruginosa .U.S. Patent Application 2011; 13/997,409
Horikawa M, Tateda K, Tuzuki E, Ishii Y, Ueda C, Takabatake T, et al. Synthesis of Pseudomonas quorum-sensing autoinducer analogs and structural entities required for induction of apoptosis in macrophages. Bioorg Med Chem Lett. 2006;16(8):2130-3.
: Hosoda H, Sakai Y, Yoshida H, Miyairi S, Ishii K, Nambara T. The preparation of steroid N-hydroxysuccinimide esters and their reactivities with bovine serum albumin. Chem Pharm Bull (Tokyo) 1979;(3):742-746.
http://dx.doi.org/10.1248/cpb.27.742
: Faezi S, Sattari M, Mahdavi M, Roudkenar MH. Passive immunisation against P.aeruginosa recombinant flagellin in an experimental model of burn wound sepsis. Burns 2011;37(5):865-72.
http://dx.doi.org/10.1016/j.burns.2010.12.003
: Haghighat S, Siadat SD, Sorkhabadi SMR, Sepahi AA, Mahdavi M. Cloning, Expression and Purification of Penicillin Binding Protein2a (PBP2a) from Methicillin Resistant Staphylococcus aureus: A Study on Immunoreactivity in Balb/C Mouse. Avicenna J Med Biotechnol 2013;5(4):204.
: Rumbaugh K P, Griswold J A, Iglewski B H, Hamood A N. Contribution of quorum sensing to the virulence of Pseudomonas aeruginosa in burn wound infections, Infect. Immun. 1999;(67):5854–5862.
: Holder IA. Pseudomonas aeruginosa virulence-associated factors and their role in burn wound infections, Pseudomonas aeruginosa: the opportunist. CRC Press, Boca Raton, FL .1993; 235-245.
: Dacheux D, Epaulard O, DeGroot A, et al. Activation of the Pseudomonas aeruginosa type III secretion system requires an intact pyruvate dehydrogenase aceAB operon. Infect. Immun. 2002; (70):3973-3977.
http://dx.doi.org/10.1128/IAI.70.7.3973-3977.2002
: Galan J E, Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science 1999(284):1322–1328.
: El-Zaim HS, Chopra AK, Peterson JW, Vasil ML, Heggers JP. Protection against Exotoxin A (ETA) and Pseudomonas aeruginosa Infection in Mice with ETA-Specific Antipeptide Antibodies. Infect. Immun. 1998;66(11):5551-4.
:Pollack M, Anderson S.E. Toxicity of pseudomonas aeruginosa exotoxin A for human macrophages. Infect.Immun 1978;(19):1092.
PMid:417028 PMCid:PMC422301
: : Furuya N, Hiralata Y, Matsumoto T, Kaker M, Yamaguchi K. Mortality rates amongst mice with endogenous septicaemia caused by Pseudomonas aeruginosa isolates from various clinical sources. J Med Microbiol. 1993(39):141.
http://dx.doi.org/10.1099/00222615-39-2-141
:Gang R K, Bang R L, Sanyal S C, Mokaddas E, Lari A R. Pseudomonas aeruginosa septicemia in burns. Burns 1999;(25): 611-612
http://dx.doi.org/10.1016/S0305-4179(99)00042-X
:Frank DW, Vallis A, Wiener-Kronish JP, Roy-Burman A, Spack EG, Mullaney BP, et al.Generation and characterization of a protective monoclonal antibody to P.aeruginosa PcrV. J.INFECT.DIS. 2002;186(1):64-73.
http://dx.doi.org/10.1086/341069
: Van Delden C, Iglewski B H. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis 1998;(4):551–560.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2015-02-08
Published 2015-05-25









