Evaluation of estrogen and G protein-coupled estrogen receptor 1 (GPER) levels in drug-naïve patients with attention deficit hyperactivity disorder (ADHD)
DOI:
https://doi.org/10.17305/bjbms.2018.2942Keywords:
Attention deficit hyperactivity disorder, ADHD, estrogen, estrogen receptors, GPER, G protein-coupled estrogen receptor 1Abstract
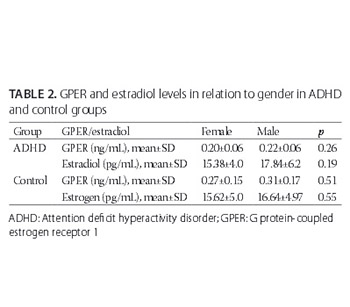
Estrogen has a crucial role in the regulation of reproductive and neuroendocrine function and exerts its effects through two classes of receptors, nuclear and membrane estrogen receptors (mERs). G protein-coupled estrogen receptor 1 (GPER) is a member of mERs, and despite limited research on the levels of GPER in patients with psychiatric diseases, a role of GPER in such conditions has been suggested. Here we evaluated serum estrogen and GPER levels in children with attention deficit hyperactivity disorder (ADHD) in relation to their age- and gender-matched healthy controls. A total of 82 children were included in the study, 47 drug- naïve patients with ADHD (age: 6–12 years; male/female: 34/13) and 35 healthy controls (age: 6–12 years; male/female: 19/16). The subgroups according to ADHD types were inattentive, hyperactive/impulsive, and combined. Serum estrogen was measured using an immunoassay system, while serum GPER was determined using a commercial sandwich enzyme-linked immunosorbent assay kit. Estrogen levels in children with ADHD were similar as in control group, while GPER levels were significantly lower in ADHD group compared to controls (p < 0.05). Logistic regression analysis showed a significant association between GPER levels and ADHD (p < 0.05), and no association between estrogen levels and ADHD (p > 0.05). No significant differences were found in GPER and estrogen levels between ADHD subgroups (p > 0.05). To the best of our knowledge, this study is the first to investigate estrogen and GPER levels in ADHD. Our preliminary findings suggest a relationship between serum GPER levels and ADHD, and this should be further investigated.
Citations
Downloads
References
Rucklidge JJ. Gender differences in attention-deficit/hyperactivity disorder. Psychiatr Clin North Am 2010; 33(2):357-73. https://doi.org/10.1016/j.psc.2010.01.006.
Hasson R, Fine JG. Gender differences among children with ADHD on continuous performance tests: A meta-analytic review. J Atten Disord 2012; 16(3):190-8. https://doi.org/10.1177/1087054711427398.
Wang LJ, Chen CK, Huang YS. Gender differences in the behavioral symptoms and neuropsychological performance of patients with attention-deficit/hyperactivity disorder treated with methylphenidate: A two-year follow-up study. J Child Adolesc Psychopharmacol 2015; 25(6):501-8. https://doi.org/10.1089/cap.2014.0175.
Martel MM, Klump K, Nigg JT, Breedlove SM, Sisk CL. Potential hormonal mechanisms of attention-deficit/hyperactivity disorder and major depressive disorder: A new perspective. Horm Behav 2009; 55(4):465-79. https://doi.org/10.1016/j.yhbeh.2009.02.004.
Davies W. Sex differences in attention deficit hyperactivity disorder: Candidate genetic and endocrine mechanisms. Front Neuroendocrinol 2014; 35(3):331-46. https://doi.org/10.1016/j.yfrne.2014.03.003.
Mueller SC, Ng P, Sinaii N, Leschek EW, Green-Golan L, VanRyzin C, et al. Psychiatric characterization of children with genetic causes of hyperandrogenism. Eur J Endocrinol 2010; 163:801-10. https://doi.org/10.1530/EJE-10-0693.
Scerbo AS, Kolko DJ. Salivary testosterone and cortisol in disruptive children: Relationship to aggressive, hyperactive, and internalizing behaviors. J Am Acad Child Adolesc Psychiatry 1994; 33(8):1174-84.
https://doi.org/10.1097/00004583-199410000-00013.
Alexander A, Irving AJ, Harvey J. Emerging roles for the novel estrogen-sensing receptor GPER1 in the CNS. Neuropharmacology 2017; 113(Pt B):652-60. https://doi.org/10.1016/j.neuropharm.2016.07.003.
Barton M. Position paper: The membrane estrogen receptor GPER – Clues and questions. Steroids 2012; 77(10):935-42. https://doi.org/10.1016/j.steroids.2012.04.001.
Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005; 307(5715):1625-30. https://doi.org/10.1126/science.1106943.
Altun I, Kurutas EB. G protein-coupled estrogen receptor levels after peripheral nerve injury in an experimental rat model. World neurosurgery 2015; 84(6):1903-6. https://doi.org/10.1016/j.wneu.2015.08.028.
Dun SL, Brailoiu GC, Gao X, Brailoiu E, Arterburn JB, Prossnitz ER, et al. Expression of estrogen receptor GPR30 in the rat spinal cord and in autonomic and sensory ganglia. J Neurosci Res 2009; 87(7):1610-9. https://doi.org/10.1002/jnr.21980.
Meyer MR, Haas E, Prossnitz ER, Barton M. Non-genomic regulation of vascular cell function and growth by estrogen. Mol Cell Endocrinol 2009; 308(1-2):9-16. https://doi.org/10.1016/j.mce.2009.03.009.
Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol 2011; 7(12):715-26. https://doi.org/10.1038/nrendo.2011.122.
Crider A, Pillai A. Estrogen signaling as a therapeutic target in neurodevelopmental disorders. J Pharmacol Exp Ther 2017;360(1):48-58. http://dx.doi.org/10.1124/jpet.116.237412.
Heringa SM, Begemann MJ, Goverde AJ, Sommer IE. Sex hormones and oxytocin augmentation strategies in schizophrenia: A quantitative review. Schizophr Res 2015;168(3):603-13. http://dx.doi.org/10.1016/j.schres.2015.04.002.
Chakrabarti B, Dudbridge F, Kent L, Wheelwright S, Hill-Cawthorne G, Allison C, et al. Genes related to sex steroids, neural growth, and social-emotional behavior are associated with autistic traits, empathy, and Asperger syndrome. Autism Res 2009; 2(3):157-77. https://doi.org/10.1002/aur.80.
Crider A, Thakkar R, Ahmed AO, Pillai A. Dysregulation of estrogen receptor beta (ERβ), aromatase (CYP19A1), and ER co-activators in the middle frontal gyrus of autism spectrum disorder subjects. Mol Autism 2014; 5(1):46.
https://doi.org/10.1186/2040-2392-5-46.
Altun H, Kurutaş EB, Şahin N, Sınır H, Fındıklı E. Decreased levels of G protein-coupled estrogen receptor in children with autism spectrum disorders. Psychiatry Res 2017; 257:67-71. https://doi.org/10.1016/j.psychres.2017.06.008.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5). USA: American Psychiatric Publishing; 2013.
Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36(7):980-8. https://doi.org/10.1097/00004583-199707000-00021.
Gökler B, Ünal F, Pehlivantürk B, Kültür EÇ, Akdemir D, Taner Y. Reliability and validity of Schedule for Affective Disorders and Schizophrenia for School Age Children-Present and Lifetime Version-Turkish Version (K-SADS-PL-T). Turk J Child Adolesc Ment Health 2004; 11(3):109-16.
Conners CK. Manual for the Conners' Rating Scales – revised. North Tonawanda, NY: Multi-Health Systems; 1997.
Kaner S, Büyüköztürk Ş, İşeri E, Ak A, Özaydın L. Validity and reliability study of the Conners' Parent Rating Scale Revised Long Form. Antalya, XVI. National Child and Adolescent Psychiatry Meeting; 2006 (abstract).
Goyette CH, Conners CK, Ulrich RF. Normative data on revised Conners' Parent and Teacher Rating Scales. J Abnorm Child Psychol 1978; 6(2):221-36. https://doi.org/10.1007/BF00919127.
Dereboy Ç, Şenol S, Şener Ş, Dereboy F. Validation of the Turkish versions of the short-form Conners' Teacher and Parent Rating Scales. [Article in Turkish]. Turk Psikiyatri Derg 2007; 18(1):48-58.
Şener S, Dereboy C, Dereboy IF, Sertcan Y. Conners' Teacher Rating Scale Turkish version-I. Turk J Child Adolesc Ment Health 1993; 2(3):131-41.
Scott E, Zhang QG, Wang R, Vadlamudi R, Brann D. Estrogen neuroprotection and the critical period hypothesis. Front Neuroendocrinol 2012; 33(1):85-104. https://doi.org/10.1016/j.yfrne.2011.10.001.
Solum DT, Handa RJ. Estrogen regulates the development of brain-derived neurotrophic factor mRNA and protein in the rat hippocampus. J Neurosci 2002; 22(7):2650-9. DOI: 20026251.
Numakawa T, Yokomaku D, Richards M, Hori H, Adachi N, Kunugi H. Functional interactions between steroid hormones and neurotrophin BDNF. World J Biol Chem 2010; 1(5):133-43. https://doi.org/10.4331/wjbc.v1.i5.133.
Yi H, Bao X, Tang X, Fan X, Xu H. Estrogen modulation of calretinin and BDNF expression in midbrain dopaminergic neurons of ovariectomised mice. J Chem Neuroanat 2016; 77:60-7. https://doi.org/10.1016/j.jchemneu.2016.05.005.
Tsai SJ. Role of neurotrophic factors in attention deficit hyperactivity disorder. Cytokine Growth Factor Rev 2017; 34:35-41. https://doi.org/10.1016/j.cytogfr.2016.11.003.
Garcia-Segura LM, Balthazart J. Steroids and neuroprotection: New advances. Front Neuroendocrinol 2009; 30(2):v-ix. https://doi.org/10.1016/j.yfrne.2009.04.006.
Inagaki T, Gautreaux C, Luine V. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Horm Behav 2010; 58(3):415-26. https://doi.org/10.1016/j.yhbeh.2010.05.013.
Watson CS, Alyea RA, Cunningham KA, Jeng YJ. Estrogens of multiple classes and their role in mental health disease mechanisms. Int J Womens Health 2010; 2:153-66. https://doi.org/10.2147/IJWH.S6907.
Mahé V, Dumaine A. Oestrogen withdrawal associated psychoses. Acta Psychiatr Scand 2001; 104(5):323-31. https://doi.org/10.1034/j.1600-0447.2001.00288.x.
Ko YH, Joe SH, Cho W, Park JH, Lee JJ, Jung IK, et al. Estrogen, cognitive function and negative symptoms in female schizophrenia. Neuropsychobiology 2006;53(4):169-75. https://doi.org/10.1159/000093780.
Hoff AL, Kremen WS, Wieneke MH, Lauriello J, Blankfeld HM, Faustman WO, et al. Association of estrogen levels with neuropsychological performance in women with schizophrenia. Am J Psychiatry 2001; 158(7):1134-9. https://doi.org/10.1176/appi.ajp.158.7.1134.
Kulkarni J, Gavrilidis E, Worsley R, Hayes E. Role of estrogen treatment in the management of schizophrenia. CNS Drugs 2012; 26(7):549-57. https://doi.org/10.2165/11630660-000000000-00000.
Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol 2011;7(12):715-26. https://doi.org/10.1038/nrendo.2011.122.
Prossnitz ER, Barton M. Signaling, physiological functions and clinical relevance of the G protein-coupled estrogen receptor GPER. Prostaglandins Other Lipid Mediat 2009; 89(3-4):89-97. https://doi.org/10.1016/j.prostaglandins.2009.05.001.
Fındıklı E, Camkurt MA, Karaaslan MF, Kurutas EB, Altun H, İzci F, et al. Serum levels of G protein-coupled estrogen receptor 1 (GPER1) in drug-naive patients with generalized anxiety disorder. Psychiatry Research 2016; 244:312-6. https://doi.org/10.1016/j.psychres.2016.04.098.
Findikli E, Kurutas EB, Camkurt MA, Karaaslan MF, Izci F, Fındıklı HA, et al. Increased serum G protein-coupled estrogen receptor 1 levels and its diagnostic value in drug naïve patients with major depressive disorder. Clin Psychopharmacol Neurosci 2017; 15(4):337-42. https://doi.org/10.9758/cpn.2017.15.4.337.
Bean LA, Ianov L, Foster TC. Estrogen receptors, the hippocampus, and memory. Neuroscientist 2014; 20(5):534-45. https://doi.org/10.1177/1073858413519865.
Lu CL, Herndon C. New roles for neuronal estrogen receptors. Neurogastroenterol Motil 2017; 29(7). https://doi.org/10.1111/nmo.13121.
Ervin KS, Mulvale E, Gallagher N, Roussel V, Choleris E. Activation of the G protein-coupled estrogen receptor, but not estrogen receptor α or β, rapidly enhances social learning. Psychoneuroendocrinology 2015;58:51-66. https://doi.org/10.1016/j.psyneuen.2015.04.002.
Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biol Psychiatry 2005; 57(11):1336-46. https://doi.org/10.1016/j.biopsych.2005.02.006.
Prossnitz ER, Barton M. Estrogen biology: New insights into GPER function and clinical opportunities. Mol Cell Endocrinol 2014; 389(1-2):71-83. https://doi.org/10.1016/j.mce.2014.02.002.
Anchan D, Clark S, Pollard K, Vasudevan N. GPR30 activation decreases anxiety in the open field test but not in the elevated plus maze test in female mice. Brain Behav 2014; 4(1):51-9. https://doi.org/10.1002/brb3.197.
Hart D, Nilges M, Pollard K, Lynn T, Patsos O, Shiel C, et al. Activation of the G-protein coupled receptor 30 (GPR30) has different effects on anxiety in male and female mice. Steroids 2014; 81:49-56. https://doi.org/10.1016/j.steroids.2013.11.004.
Downloads
Additional Files
Published
How to Cite
Accepted 2018-02-20
Published 2018-05-20









