The role of lipid dysregulation and vascular risk factors in glaucomatous retrobulbar circulation
DOI:
https://doi.org/10.17305/bjbms.2015.299Keywords:
primary open angle glaucoma, retrobulbar circulation, color Doppler imaging, vascular and lipid-related risk factors, ocular hemodynamicsAbstract
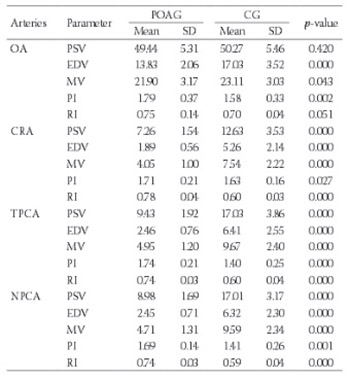
The aim of this study was to evaluate selected lipid-related and vascular factors and their effect on retrobulbar hemodynamics in glaucoma. Fifty-six patients with primary open angle glaucoma (POAG) [POAG group; mean age 68.32 years (SD±0.21)] and 54 patients in control group [CG, mean age 68.1 years (SD±5.34)] were examined. Peak systolic velocity, end-diastolic velocity, mean velocity, pulsatility index, and resistive index of the ophthalmic artery, the central retinal artery and the posterior ciliary arteries were measured by Color Doppler Imaging. Selected lipid-related, systemic and local vascular parameters were evaluated. Statistical methods included Shapiro-Wilk, Student-t and Mann-Whitney U tests, and Spearman rank correlations. In POAG group systolic arterial blood pressure, diastolic arterial blood pressure, total cholesterol, low density lipoprotein cholesterol (LDL-ch), and intraocular pressure were significantly higher; while ocular perfusion pressure, high density lipoprotein cholesterol (HDL-ch) and diastolic ocular perfusion pressure were significantly lower (p≤0.05). Color Doppler Imaging confirmed blood flow abnormalities in all investigated arteries. In addition, significant correlations of HDL-ch, LDL-ch and triglycerides (TG) with peak systolic velocity, end-diastolic velocity and mean velocity were found in individual arteries (p≤0.05). Also, significant associations of systolic arterial blood pressure, ocular perfusion pressure, systolic oclular perfusion pressure and diastolic ocular perfusion pressure with peak systolic velocity, end-diastolic velocity, mean velocity and resistive index were revealed in the posterior ciliary arteries (p≤0.05). Dysregulation of lipid-related and vascular factors, as well as statistical correlation between the above and retrobulbar blood flow indices, might imply their role in vasoconstrictive processes during glaucomatous endotheliopathy.
Citations
Downloads
References
Bonomi L, Marchini G, Marraffa M, Bernardi P, Morbio R, Varotto A. Vascular risk factors for primary open angle glaucoma: The Egna-Neumarkt Study. Ophthalmology. 2000;107(7):1287–93.
http://dx.doi.org/10.1016/S0161-6420(00)00138-X
Harris A, Evans D, Martin B, Zalish M, Kagemann L, McCranor L, et al. Nocturnal blood pressure reduction: effect on retrobulbar hemodynamics in glaucoma. Graefes Arch Clin Exp Ophthalmol. 2002;240(5):372–8.
http://dx.doi.org/10.1007/s00417-002-0466-y
Flammer J, Haefliger IO, Orgül S, Resink T. Vascular dysregulation: A principal risk factor for glaucomatous damage? J Glaucoma. 1999;8(3):212–9.
http://dx.doi.org/10.1097/00061198-199906000-00012
Martinez A, Sanchez M. Predictive value of colour Doppler imaging in a prospective study of visual field progression in primary open-angle glaucoma. Acta Ophthalmol Scand. 2005;83(6):716–22.
http://dx.doi.org/10.1111/j.1600-0420.2005.00567.x
Carter CJ, Brooks DE, Doyle DL, Drance SM. Investigations into a vascular etiology for low-tension glaucoma. Ophthalmology. 1990;97(1):49–55.
http://dx.doi.org/10.1016/S0161-6420(90)32627-1
Dewey M. Comprehensive assessment of peripheral artery disease using magnetic resonance imaging, angiography, and spectroscopy. J Am Coll Cardiol. 2009;54(7):636–7.
http://dx.doi.org/10.1016/j.jacc.2009.04.055
Choi J, Joe SG, Seong M, Choi JY, Sung KR, Kook MS. C-reactive protein and lipid profiles in Korean patients with normal tension glaucoma. Korean J Ophthalmol. 2009;23(3):193–7.
http://dx.doi.org/10.3341/kjo.2009.23.3.193
Stewart WC, Osterman J. Serum lipid physiology and the influence of glaucoma medications. Surv Ophthalmol. 1998;43(3):233–44.
http://dx.doi.org/10.1016/S0039-6257(98)00030-7
Deokule S, Vizzeri G, Boehm AG, Bowd C, Medeiros FA, Weinreb RN. Correlation among choroidal, parapapillary, and retrobulbar vascular parameters in glaucoma. Am J Ophthalmol. 2009;147(4):736–43.e2.
http://dx.doi.org/10.1016/j.ajo.2008.10.020
Wong TY, Klein R, Sharrett AR, Nieto FJ, Boland LL, Couper DJ, et al. Retinal microvascular abnormalities and cognitive impairment in middle-aged persons. The atherosclerosis risk in communities study. Stroke. 2002;33(6):1487–92.
http://dx.doi.org/10.1161/01.STR.0000016789.56668.43
Modrzejewska M, Ostanek L, Bobrowska-Snarska D, Karczewicz D, Wilk G, Brzosko M, et al. Ocular circulation in systemic lupus erythematosus. Med Sci Monit. 2009;15(11):CR573–8.
Modrzejewska M, Pieńkowska-Machoy E, Grzesiak W, Karczewicz D, Wilk G. Predictive value of color Doppler imaging in an evaluation of retrobulbar blood flow perturbation in young type-1 diabetic patients with regard to dyslipidemia. Med Sci Monit. 2008;14(10):MT47–52.
Harris A, Arend O, Kopecky K, Caldemeyer K, Wolf S, Sponsel W, et al. Physiological perturbation of ocular and cerebral blood flow as measured by scanning laser ophthalmoscopy and color Doppler imaging. Surv Ophthalmol. 1994;38 Suppl:S81–6.
http://dx.doi.org/10.1016/0039-6257(94)90050-7
Dimitrova G, Kato S. Color Doppler imaging of retinal diseases. Surv Ophthalmol. 2010;55(3):193–214.
http://dx.doi.org/10.1016/j.survophthal.2009.06.010
Topouzis F, Coleman AL, Harris A, Jonescu-Cuypers C, Yu F, Mavroudis L, et al. Association of blood pressure status with the optic disk structure in non-glaucoma subjects: the Thessaloniki eye study. Am J Ophthalmol. 2006;142(1):60–7.
http://dx.doi.org/10.1016/j.ajo.2006.02.055
Harris A, Januleviciene I, Siesky B, Schmetterer L, Kageman L, Stalmans I, et al. Clinical measurement of ocular blood flow. In; Weinreb RN Harris A (eds). Ocul. Blood Flow Glaucoma 6th Consens. Rep. World Glaucoma Assoc., Amsterdam: Kugler Publications; 2009.
Stalmans I, Vandewalle E, Anderson DR, Costa VP, Frenkel RE, Garhofer G, et al. Use of colour Doppler imaging in ocular blood flow research. Acta Ophthalmol. 2011;89(8):e609–30.
http://dx.doi.org/10.1111/j.1755-3768.2011.02178.x
Güngör İU, Güngör L, Özarslan Y, Arıtürk N, Beden Ü, Erkan D, et al. Is symptomatic atherosclerotic cerebrovascular disease a risk factor for normal-tension glaucoma? Med Princ Pract. 2011;20(3):220–4.
http://dx.doi.org/10.1159/000323596
Pavljašević S, Ašćerić M. Primary open-angle glaucoma and serum lipids. Bosn J Basic Med Sci. 2009;9(1):85–8.
Martin M, Hulley SB, Browner WS, Kuller LH, Wentworth D. Serum cholesterol, blood pressure, and mortality: implications from a cohort of 361,662 men. Lancet. 1986;2(8513):933–6.
http://dx.doi.org/10.1016/S0140-6736(86)90597-0
Gasser P, Flammer J. Blood-cell velocity in the nailfold capillaries of patients with normal-tension or high-tension glaucoma. Am J Ophthalmol. 1991;111(5):585–8.
http://dx.doi.org/10.1016/S0002-9394(14)73703-1
Flammer J. The vascular concept of glaucoma. Surv Ophthalmol. 1994;38 Suppl:S3–6.
http://dx.doi.org/10.1016/0039-6257(94)90041-8
Klein BE, Klein R, Linton KL, Franke T. Cigarette smoking and lens opacities: the Beaver Dam Eye Study. Am J Prev Med. 1993;9(1):27-30.
Hayreh SS, Bill A, Sperber GO. Effects of high intraocular pressure on the glucose metabolism in the retina and optic nerve in old atherosclerotic monkeys. Graefes Arch Clin Exp Ophthalmol. 1994;232(12):745–52.
http://dx.doi.org/10.1007/BF00184278
Grunwald JE, Piltz J, Hariprasad SM, Dupont J, Maguire MG. Optic nerve blood flow in glaucoma: Effect of systemic hypertension. Am J Ophthalmol. 1999;127(5):516–22.
http://dx.doi.org/10.1016/S0002-9394(99)00028-8
Pache M, Dubler B, Flammer J. Peripheral vasospasm and nocturnal blood pressure dipping–two distinct risk factors for glaucomatous damage? Eur J Ophthalmol. 2003;13(3):260–5.
Su WW, Cheng ST, Ho WJ, Tsay PK, Wu SC, Chang SH. Glaucoma is associated with peripheral vascular endothelial dysfunction. Ophthalmology. 2008;115(7):1173–8.e1.
http://dx.doi.org/10.1016/j.ophtha.2007.10.026
Haefliger IO, Meyer P, Flammer J, Lüscher TF. The vascular endothelium as a regulator of the ocular circulation: A new concept in ophthalmology? Surv Ophthalmol. 1994;39(2):123–32.
http://dx.doi.org/10.1016/0039-6257(94)90157-0
Schmetterer L, Polak K. Role of nitric oxide in the control of ocular blood flow. Prog Retin Eye Res. 2001;20(6):823–47.
http://dx.doi.org/10.1016/S1350-9462(01)00014-3
Cohen R, Padilla J, Light D, Diller R. Carotid artery occlusive disease and ocular manifestations: Importance of identifying patients at risk. Optometry. 2010;81(7):359–63.
http://dx.doi.org/10.1016/j.optm.2009.10.013
Shoji T, Sakurai Y, Sato H, Chihara E, Ishida M, Omae K. Serum low-density lipoprotein cholesterol level is strong risk factor for acquired color vision impairment in young to middle-aged Japanese men: The Okubo Color Study Report 2. Atherosclerosis. 2010;210(2):542–7.
http://dx.doi.org/10.1016/j.atherosclerosis.2009.11.039
Haefliger IO, Dettmann E, Liu R, Meyer P, Prünte C, Messerli J, et al. Potential role of nitric oxide and endothelin in the pathogenesis of glaucoma. Surv Ophthalmol. 1999;43 Suppl 1:S51–8.
http://dx.doi.org/10.1016/S0039-6257(99)00026-0
Emre M, Orgül S, Gugleta K, Flammer J. Ocular blood flow alteration in glaucoma is related to systemic vascular dysregulation. Br J Ophthalmol. 2004;88(5):662–6.
http://dx.doi.org/10.1136/bjo.2003.032110
Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–6.
http://dx.doi.org/10.1038/288373a0
Zhu P, Dettmann ES, Resink TJ, Lüscher TF, Flammer J, Haefliger IO. Effect of Ox-LDL on endothelium-dependent response in pig ciliary artery: Prevention by an ET (A) antagonist. Invest Ophthalmol Vis Sci. 1999;40(5):1015–20.
Resch H, Garhofer G, Fuchsjäger-Mayrl G, Hommer A, Schmetterer L. Endothelial dysfunction in glaucoma. Acta Ophthalmol. 2009;87(1):4–12.
http://dx.doi.org/10.1111/j.1755-3768.2007.01167.x
Rosenthal R, Fromm M. Endothelin antagonism as an active principle for glaucoma therapy. Br J Pharmacol. 2011;162(4):806–16.
http://dx.doi.org/10.1111/j.1476-5381.2010.01103.x
Babizhayev MA, Bunin AY. Lipid peroxidation in open-angle glaucoma. Acta Ophthalmol (Copenh). 1989;67(4):371–7.
http://dx.doi.org/10.1111/j.1755-3768.1989.tb01617.x
Garhöfer G, Fuchsjäger-Mayrl G, Vass C, Pemp B, Hommer A, Schmetterer L. Retrobulbar blood flow velocities in open angle glaucoma and their association with mean arterial blood pressure. Invest Ophthalmol Vis Sci. 2010;51(12):6652–7.
http://dx.doi.org/10.1167/iovs.10-5490
Jonas JB, Fernandez MC, Naumann GO. Parapapillary atrophy and retinal vessel diameter in nonglaucomatous optic nerve damage. Invest Ophthalmol Vis Sci. 1991;32(11):2942–7.
Rader J, Feuer WJ, Anderson DR. Peripapillary vasoconstriction in the glaucomas and the anterior ischemic optic neuropathies. Am J Ophthalmol. 1994;117(1):72–80.
http://dx.doi.org/10.1016/S0002-9394(14)73017-X
Kerr J, Nelson P, O'Brien C. A comparison of ocular blood flow in untreated primary open-angle glaucoma and ocular hypertension. Am J Ophthalmol. 1998;126(1):42–51.
http://dx.doi.org/10.1016/S0002-9394(98)00074-9
Modrzejewska M, Karczewicz D, Wilk G. Use of color Doppler ultrasonography in primary vasospastic syndrome and assessment of ocular blood flow in patients with transient monocular blindness. Pol J Radiol. 2007;72(2):9–14.
Harris A, Kagemann L, Ehrlich R, Rospigliosi C, Moore D, Siesky B. Measuring and interpreting ocular blood flow and metabolism in glaucoma. Can J Ophthalmol. 2008;43(3):328–36.
http://dx.doi.org/10.1139/I08-051
Galassi F, Sodi A, Ucci F, Renieri G, Pieri B, Baccini M. Ocular hemodynamics and glaucoma prognosis: A color Doppler imaging study. Arch Ophthalmol. 2003;121(12):1711–5.
http://dx.doi.org/10.1001/archopht.121.12.1711
Swietliczko I, David NJ. Fluorescein angiography in experimental ocular hypertension. Am J Ophthalmol. 1970;70(3):351–63.
http://dx.doi.org/10.1016/0002-9394(70)90094-2
Spencer JA, Giussani DA, Moore PJ, Hanson MA. In vitro validation of Doppler indices using blood and water. J Ultrasound Med. 1991;10(6):305–8.
Steigerwalt RD Jr, Laurora G, Belcaro GV, Cesarone MR, De Sanctis MT, Incandela L, et al. Ocular and retrobulbar blood flow in ocular hypertensives treated with topical timolol, betaxolol and carteolol. J Ocul Pharmacol Ther. 2001;17(6):537–44.
http://dx.doi.org/10.1089/10807680152729220
Collignon NJ, Collignon-Brach JD. Effect of topical beta blockers on human retinal vessels diameters. Int Ophthalmol. 1997-1998;21(4):199–203.
http://dx.doi.org/10.1023/A:1005918922700
Gugleta K. [Vascular risk factors in glaucoma-diagnostics]. Praxis (Bern 1994). 2009;98(4):201–7. German.
Tielsch JM, Katz J, Sommer A, Quigley HA, Javitt JC. Hypertension, perfusion pressure, and primary open-angle glaucoma. A population-based assessment. Arch Ophthalmol. 1995;113(2):216–21.
http://dx.doi.org/10.1001/archopht.1995.01100020100038
Quigley HA, West SK, Rodriguez J, Munoz B, Klein R, Snyder R. The prevalence of glaucoma in a population-based study of Hispanic subjects: Proyecto VER. Arch Ophthalmol. 2001;119(12):1819–26.
http://dx.doi.org/10.1001/archopht.119.12.1819
Leske MC, Connell AM, Wu SY, Nemesure B, Li X, Schachat A, et al. Incidence of open-angle glaucoma: the Barbados Eye Studies. The Barbados Eye Studies Group. Arch Ophthalmol. 2001;119(1):89–95.
Leske MC, Wu SY, Nemesure B, Hennis A. Incident open-angle glaucoma and blood pressure. Arch Ophthalmol. 2002;120(7):954–9.
http://dx.doi.org/10.1001/archopht.120.7.954
Choi J, Jeong J, Cho HS, Kook MS. Effect of nocturnal blood pressure reduction on circadian fluctuation of mean ocular perfusion pressure: a risk factor for normal tension glaucoma. Invest Ophthalmol Vis Sci. 2006;47(3):831–6.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2015-02-09
Published 2015-03-15









