Dose fall-off during the treatment of thoracic spine metastasis with CyberKnife stereotactic body radiation therapy (SBRT)
DOI:
https://doi.org/10.17305/bjbms.2018.3185Keywords:
CyberKnife, dosage, radiation, stereotactic body radiation therapy, SBRT, thoracic spine metastasis, dose gradientAbstract
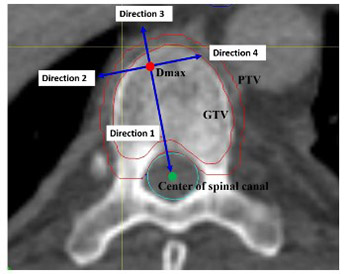
CyberKnife stereotactic body radiation therapy (SBRT) is becoming increasingly used for cancer treatment and, to maximize its clinical application, it is important to define the dosimetric characteristics, optimal dose, and fractionation regimens. The aim of this study was to evaluate the dose fall-off in two fractionated regimens of CyberKnife SBRT during the treatment of thoracic spinal metastasis. Patients with spinal metastasis involving a vertebra and pedicle were treated with 40 Gy in 5 fractions (n = 4), and patients with spinal metastasis involving only a vertebra received 33 Gy in 3 fractions (n = 4). A new approach was used to measure absolute dose fall-off distance, relative dose fall-off distance, and the dose fall-off per unit distance along four reference directions in the axial plane. Patients treated with 33 Gy/3 fractions had a greater absolute dose fall-off distance in direction 1 (from the point with maximum dose [Dmax] towards the spinal cord) and direction 3 (the opposite of direction 1), a greater relative dose fall-off distance in direction 3, and a lower dose fall-off per unit distance in direction 1 and 3 compared to patients treated with 40 Gy/5 fractions (all p < 0.05). Overall, the dose fall-off towards the spinal cord is rapid during the treatment of thoracic spinal metastasis with CyberKnife SBRT, which allows a higher dose of radiation to be delivered to the tumor and, at the same time, better protection of the spinal cord.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2018-07-18
Published 2020-02-05









