NOD2/CARD15 mutations in Polish and Bosnian populations with and without Crohn's disease: prevalence and genotype-phenotype analysis
DOI:
https://doi.org/10.17305/bjbms.2015.348Keywords:
NOD2/CARD15 gene, Crohn's disease, genotype-phenotype analysis, gene frequency, Poland, Bosnia and HerzegovinaAbstract
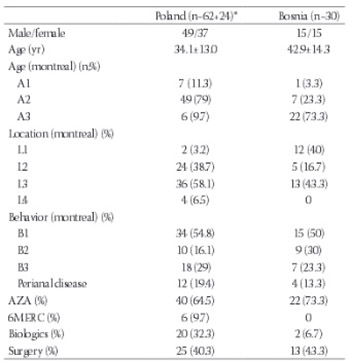
Data on prevalence and phenotypic consequences of nucleotide-binding oligomerization domain 2/caspase recruitment domains 15 (NOD2/CARD15) variants in Crohn's disease (CD) population in Poland and Bosnia and Herzegovina (BH) are nonexistent. We aimed to determine the prevalence of NOD2/CARD15 mutations and their association with disease phenotype in Polish and Bosnian patients with CD and in healthy controls. We prospectively recruited 86 CD patients and 83 controls in Poland and 30 CD patients and 30 controls in BH, 229 in total. We determined the prevalence of NOD2/CARD15 mutations and their association with the disease phenotype according to Montreal classification. Participants were genotyped for Leu1007fsinsC and Gly908Arg mutations. At least one CD-associated allele was found in 29/86 (33.7%) of Polish CD patients and in 9/83 (10.8%) of healthy controls (p<0.001). In both CD patients and controls in the Bosnian sample, at least one NOD2 mutation was found in an equal number of patients (3/30; 10%) with all of the NOD2 mutation-positive CD patients being homozygous, while controls being heterozygous. In the Polish sample, the perianal disease was less frequent in CD patients with any NOD2 mutation (1/21; 4.8%) compared to those without (11/41; 26.8%; p=0.046). The higher percentage of patients with NOD2 mutations had a history of CD related surgery when compared with those without mutations (66.7% vs. 43.3%; p=0.05). The risk for CD is increased in patients with NOD2 mutations (Poland) and especially in the presence of homozygous NOD2 mutations (Poland and Bosnia). The presence of variant NOD2 alleles is associated with an increased need for surgery and reduced occurrence of perianal disease.
Citations
Downloads
References
Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn's disease: Definitions and diagnosis. Journal of Crohn's & colitis. 2010;4:7-27.
http://dx.doi.org/10.1016/j.crohns.2009.12.003
Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, et al. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nature genetics. 2008;40:955-62.
http://dx.doi.org/10.1038/ng.175
Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603-6.
http://dx.doi.org/10.1038/35079114
Alvarez-Lobos M, Arostegui JI, Sans M, Tassies D, Plaza S, Delgado S, et al. Crohn's disease patients carrying NOD2/CARD15 gene variants have an increased and early need for first surgery due to stricturing disease and higher rate of surgical recurrence. Annals of surgery. 2005;242:693-700.
http://dx.doi.org/10.1097/01.sla.0000186173.14696.ea
Angelberger S, Reinisch W, Dejaco C, Miehsler W, Waldhoer T, Wehkamp J, et al. NOD2/CARD15 gene variants are linked to failure of antibiotic treatment in perianal fistulating Crohn's disease. Am J Gastroenterol. 2008;103:1197-202.
http://dx.doi.org/10.1111/j.1572-0241.2007.01741.x
Bianchi V, Maconi G, Ardizzone S, Colombo E, Ferrara E, Russo A, et al. Association of NOD2/CARD15 mutations on Crohn's disease phenotype in an Italian population. Eur J Gastroenterol Hepatol. 2007;19:217-23.
http://dx.doi.org/10.1097/01.meg.0000250590.84102.12
Gazouli M, Zacharatos P, Mantzaris GJ, Barbatis C, Ikonomopoulos I, Archimandritis AJ, et al. Association of NOD2/CARD15 variants with Crohn's disease in a Greek population. Eur J Gastroenterol Hepatol. 2004;16:1177-82.
http://dx.doi.org/10.1097/00042737-200411000-00016
Hugot J-P, Chamaillard M, Zouali H, Lesage S, Cezard J-P, Belaiche J, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599-603.
http://dx.doi.org/10.1038/35079107
Lauriola M, Ugolini G, Rivetti S, Nani S, Rosati G, Zanotti S, et al. IL23R, NOD2/CARD15, ATG16L1 and PHOX2B polymorphisms in a group of patients with Crohn's disease and correlation with sub-phenotypes. International journal of molecular medicine. 2011;27:469-77.
http://dx.doi.org/10.3892/ijmm.2010.591
Hampe J, Cuthbert A, Croucher PJ, Mirza MM, Mascheretti S, Fisher S, et al. Association between insertion mutation in NOD2 gene and Crohn's disease in German and British populations. Lancet. 2001;357:1925-8.
http://dx.doi.org/10.1016/S0140-6736(00)05063-7
Bonen DK, Cho JH. The genetics of inflammatory bowel disease. Gastroenterology. 2003;124:521-36.
http://dx.doi.org/10.1053/gast.2003.50045
Lesage S, Zouali H, Cezard JP, Colombel JF, Belaiche J, Almer S, et al. CARD15/NOD2 mutational analysis and genotype-phenotype correlation in 612 patients with inflammatory bowel disease. American journal of human genetics. 2002;70:845-57.
http://dx.doi.org/10.1086/339432
Vermeire S, Wild G, Kocher K, Cousineau J, Dufresne L, Bitton A, et al. CARD15 genetic variation in a Quebec population: prevalence, genotype-phenotype relationship, and haplotype structure. American journal of human genetics. 2002;71:74-83.
http://dx.doi.org/10.1086/341124
Radlmayr M, Torok HP, Martin K, Folwaczny C. The c-insertion mutation of the NOD2 gene is associated with fistulizing and fibrostenotic phenotypes in Crohn's disease. Gastroenterology. 2002;122:2091-2.
http://dx.doi.org/10.1053/gast.2002.34020
Helio T, Halme L, Lappalainen M, Fodstad H, Paavola-Sakki P, Turunen U, et al. CARD15/NOD2 gene variants are associated with familially occurring and complicated forms of Crohn's disease. Gut. 2003;52:558-62.
http://dx.doi.org/10.1136/gut.52.4.558
Buning C, Genschel J, Buhner S, Kruger S, Kling K, Dignass A, et al. Mutations in the NOD2/CARD15 gene in Crohn's disease are associated with ileocecal resection and are a risk factor for reoperation. Aliment Pharmacol Ther. 2004;19:1073-8.
http://dx.doi.org/10.1111/j.1365-2036.2004.01967.x
Abreu MT, Taylor KD, Lin YC, Hang T, Gaiennie J, Landers CJ, et al. Mutations in NOD2 are associated with fibrostenosing disease in patients with Crohn's disease. Gastroenterology. 2002;123:679-88.
http://dx.doi.org/10.1053/gast.2002.35393
Bhullar M, Macrae F, Brown G, Smith M, Sharpe K. Prediction of Crohn's disease aggression through NOD2/CARD15 gene sequencing in an Australian cohort. World J Gastroenterol. 2014;20:5008-16.
http://dx.doi.org/10.3748/wjg.v20.i17.5008
Lovasz BD, Golovics PA, Vegh Z, Lakatos PL. New trends in inflammatory bowel disease epidemiology and disease course in Eastern Europe. Digestive and Liver Disease. 2013;45:269-76.
http://dx.doi.org/10.1016/j.dld.2012.08.020
Pavlovic-Calic N, Salkic NN, Gegic A, Smajic M, Alibegovic E. Crohn's disease in Tuzla region of Bosnia and Herzegovina: a 12-year study (1995-2006). International journal of colorectal disease. 2008;23:957-64.
http://dx.doi.org/10.1007/s00384-008-0493-1
Wiercinska-Drapalo A, Jaroszewicz J, Flisiak R, Prokopowicz D. Epidemiological characteristics of inflammatory bowel disease in North-Eastern Poland. World J Gastroenterol. 2005;11:2630-3.
http://dx.doi.org/10.3748/wjg.v11.i17.2630
Cukovic-Cavka S, Vermeire S, Hrstic I, Claessens G, Kolacek S, Jakic-Razumovic J, et al. NOD2/CARD15 mutations in Croatian patients with Crohn's disease: prevalence and genotype-phenotype relationship. Eur J Gastroenterol Hepatol. 2006;18:895-9.
http://dx.doi.org/10.1097/00042737-200608000-00016
Protic MB, Pavlovic ST, Bojic DZ, Krstic MN, Radojicic ZA, Tarabar DK, et al. CARD15 gene polymorphisms in Serbian patients with Crohn's disease: genotype-phenotype analysis. Eur J Gastroenterol Hepatol. 2008;20:978-84.
http://dx.doi.org/10.1097/MEG.0b013e328302f45e
Hradsky O, Lenicek M, Dusatkova P, Bronsky J, Nevoral J, Valtrova V, et al. Variants of CARD15, TNFA and PTPN22 and susceptibility to Crohn's disease in the Czech population: high frequency of the CARD15 1007fs. Tissue antigens. 2008;71:538-47.
http://dx.doi.org/10.1111/j.1399-0039.2008.01047.x
Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749-53.
http://dx.doi.org/10.1136/gut.2005.082909
Hosek J, Bartosova L, Gregor P, Kolorz M, Dite P, Batovsky M, et al. Frequency of representative single nucleotide polymorphisms associated with inflammatory bowel disease in the Czech Republic and Slovak Republic. Folia biologica. 2008;54:88-96.
Ludwika Jakubowska-Burek, Elzbieta Kaczmarek, Justyna Hoppe-Golebiewska, Marta Kaczmarek-Rys, Szymon Hryhorowicz, Marcin A. Kucharski, et al. Genotyping of CARD15/NOD2, ATG16L1 and IL23R Genes in Polish Crohn's Disease (CD) Patients – Are They Related to the Localization of the Disease and Extra-Intestinal Symptoms? In: Karoui S, editor. Crohn's Disease: InTech; 2012. p. 39-57.
http://dx.doi.org/10.5772/1170
Fernandez-Salazar LI, Gomez-Gonzalez E, Velayos B, Barrio J, Gonzalez JM, Arranz E, et al. NOD2/CARD15 gene mutations in patients with inflammatory bowel disease in Valladolid. Revista espanola de enfermedades digestivas : organo oficial de la Sociedad Espanola de Patologia Digestiva. 2011;103:500.
http://dx.doi.org/10.4321/S1130-01082011000900016
Long WY, Chen L, Zhang CL, Nong RM, Lin MJ, Zhan LL, et al. Association between NOD2/CARD15 gene polymorphisms and Crohn's disease in Chinese Zhuang patients. World J Gastroenterol. 2014;20:4737-44.
http://dx.doi.org/10.3748/wjg.v20.i16.4737
Meddour Y, Chaib S, Bousseloub A, Kaddache N, Kecili L, Gamar L, et al. NOD2/CARD15 and IL23R genetic variability in 204 Algerian Crohn's disease. Clin Res Hepatol Gastroenterol. 2014.
Radford-Smith G, Pandeya N. Associations between NOD2/CARD15 genotype and phenotype in Crohn's disease--Are we there yet? World J Gastroenterol. 2006;12:7097-103.
Adler J, Rangwalla SC, Dwamena BA, Higgins PD. The prognostic power of the NOD2 genotype for complicated Crohn's disease: a meta-analysis. Am J Gastroenterol. 2011;106:699-712.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2015-02-23
Published 2015-05-25









