Vascular endothelial growth factor (VEGF)-related polymorphisms rs10738760 and rs6921438 are not risk factors for proliferative diabetic retinopathy (PDR) in patients with type 2 diabetes mellitus (T2DM)
DOI:
https://doi.org/10.17305/bjbms.2018.3519Keywords:
Vascular endothelial growth factor, VEGF, SNP, single nucleotide polymorphism, proliferative diabetic retinopathy, PDR, T2DM, diabetes, VEGFR-2Abstract
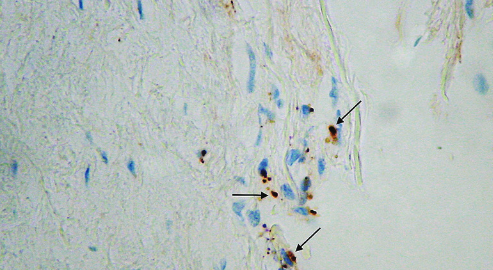
Vascular endothelial growth factor (VEGF) is an important regulator of angiogenesis and has been investigated as a candidate gene in a number of conditions, including diabetes and its microvascular complications (e.g., retinopathy and nephropathy). Several VEGF-related polymorphisms have been shown to contribute to nearly half of the variability in circulating VEGF levels in healthy individuals. Our aim was to assess the association between VEGF-related rs10738760 and rs6921438 polymorphisms and proliferative diabetic retinopathy (PDR) in Slovenian patients with type 2 diabetes mellitus (T2DM). We also investigated the effect of these polymorphisms on VEGF receptor 2 (VEGFR-2) expression in fibrovascular membranes (FVMs) from patients with PDR. This case-control study enrolled 505 unrelated patients with T2DM: 143 diabetic patients with PDR as a study group, and 362 patients with T2DM of >10 years duration and with no clinical signs of PDR as a control group. Patient clinical and laboratory data were obtained from their medical records. rs10738760 and rs6921438 polymorphisms were genotyped using TaqMan SNP Genotyping assay. VEGFR-2 expression was assessed by immunohistochemistry in 20 FVMs from patients with PDR, and numerical areal density of VEGFR-2-positive cells was calculated. The occurrence of PDR was 1.7 times higher in diabetic patients carrying GA genotype of rs6921438 compared to patients with GG genotype, with a borderline statistical significance (OR = 1.7, 95% CI = 1.00 – 2.86, p = 0.05). In addition, A allele of rs6921438 was associated with increased VEGFR-2 expression in FVMs from PDR patients. However, we observed no association between AA genotype of rs6921438 nor between rs10738760 variants and PDR, indicating that the two polymorphisms are not genetic risk factors for PDR.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2018 Bosnian Journal of Basic Medical Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2018-07-19
Published 2019-02-12









