Isolation and immunochemical characterization of human cystatin C
DOI:
https://doi.org/10.17305/bjbms.2002.3576Keywords:
human cystatin C, cystatin C isoforms, ELISA test, chronic renal failureAbstract
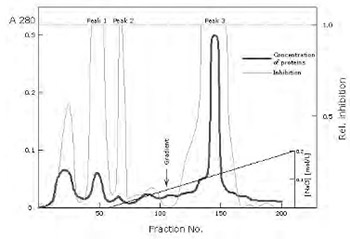
Cystatin C is a natural inhibitor of the cysteine proteinases papain, and mammalian lysosomal cathepsins B, H, L and S. This protein is thought to serve an important physiological role as a local regulator of enzyme activity. The changes of levels of cystatin C in extracellular fluids have shown themselves having potential clinical importance. We have purified cystatin C from urine of patients with chronic renal failure by procedure using affinity chromatography on CM-papain Sepharose, gel filtration on Sephacryl S-200, and ion exchange chromatography on CM-cellulose. After isolation we obtained three inhibitory peaks (pI's from 7.8 to 9.2) which represent isoforms of the same protein. These isoforms are immunologically identical and differ in N-terminal sequence of the molecule. The form with pI 9.2 represents the intact inhibitor form, whereas the form with pI 7.8 is shortened for 8 amino-acid residues at N-terminal end. Purified cystatin C pI 9.2 was used for immunization of rabbits. Polyclonal antibodies, produced in rabbits, were isolated from rabbit sera by affinity chromatography on Protein A Sepharose. Enzyme immunoassay (ELISA) for cystatin C is developed on the basis of purified antibodies. Using ELISA test we determined amount of cystatin C in urine and serum samples of patients with chronic renal failure. The concentration of the inhibitor in the urine of these patients was approximately 100-fold more than in normal urine. In the serum from the same patients we found concentrations of cystatin C to be five times higher in comparison with the serum of healthy individuals.
Citations
Downloads
Published
Issue
Section
Categories
How to Cite
Accepted 2018-05-05
Published 2002-02-20









