Wound-healing potential of the fruit extract of Phaleria macrocarpa
DOI:
https://doi.org/10.17305/bjbms.2015.39Keywords:
Antioxidant enzyme, inflammatory mediator, Phaleria macrocarpa, wound healingAbstract
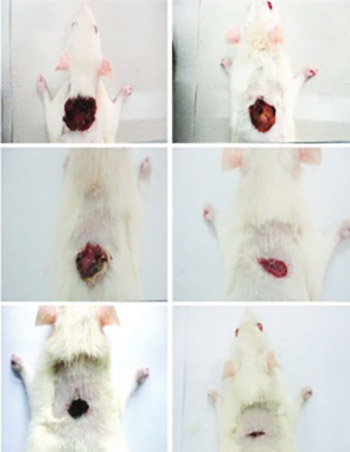
The wound-healing potential of Phaleria macrocarpa was evaluated by monitoring the levels of inflammatory mediators, collagen, and antioxidant enzymes. Experimentally, two-centimeter-wide full-thickness-deep skin excision wounds were created on the posterior neck area of the rats. The wounds were topically treated with gum acacia as a vehicle in the control group, intrasite gel in the reference group, and 100 and 200 mg/mL P. macrocarpa fruit extract in the treatment group. Granulation tissues were excised on the 15th day and were further processed for histological and biochemical analyzes. Wound healing was evaluated by measuring the contractions and protein contents of the wounds. Cellular redistribution and collagen deposition were assessed morphologically using Masson’s trichrome stain. Superoxide dismutase (SOD) and catalase (CAT) activities, along with malondialdehyde (MDA) level were determined in skin tissue homogenates of the dermal wounds. Serum levels of transforming growth factor beta 1 (TGF-β1) and tumor necrosis factor alpha (TNF-α) were evaluated in all the animals. A significant decrease in wound area was caused by a significant increase in TGF-β1 level in the treated groups. Decrease in TNF-α level and increase in the collagen formation were also observed in the treated groups. Topical treatment with P. macrocarpa fruit extract increased the SOD and CAT activities in the healing wounds, thereby significantly increasing MDA level. The topical treatment with P. macrocarpa fruit extract showed significant healing effect on excision wounds and demonstrated an important role in the inflammation process by increasing antioxidant enzyme activities, thereby accelerating the wound healing process and reducing tissue injury.
Citations
Downloads
References
Boateng JS, Matthews KH, Stevens HN, Eccleston GM. Wound healing dressings and drug delivery systems: a review. J Pharm Sci 2008;97(8):2892-2923. DOI: 10.1002/jps.21210.
Mackay D, Miller AL. Nutritional Support for Wound Healing. Alter Med Rev 2003;8(4):359-377.
Bullard KM, Lund L, Mudgett JS, Mellin TN, Hunt TK, Murphy B, et al. Impaired wound contraction in stromelysin-1–deficient mice. Ann Surg 1999;230(2):260-265. DOI: 10.1097/00000658-199908000-00017.
Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci. 2004;9(1):283-289. DOI:10.2741/1184.
Menke NB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF. Impaired wound healing. Clin Dermatol 2007;25(1):19-25. DOI: 10.1016/j.clindermatol.2006.12.005.
Schwentker A, Vodovotz Y, Weller R, Billiar TR. Nitric oxide and wound repair: role of cytokines? Nitric oxide 2002;7(1):1-10. DOI:10.1016/S1089-8603(02)00002-2.
Guo S, Dipietro LA. Factors affecting wound healing.J Dent Res 2010;89(3):219-229.
DOI: 10.1177/0022034509359125.
Hendra R, Ahmad S, Sukari A, Shukor MY, Oskoueian E. Flavonoid analyses and antimicrobial activity of various parts of Phaleria macrocarpa (Scheff.) boerl fruit. Int J Mol Sci 2011;12(6):3422-3431. DOI: 10.3390/ijms12063422.
Soo C, Chong, Mohamad A, Dollah, Pei PC, Abdullah M. Phaleria macrocarpa (Scheff.) Boerl fruit aqueous extract enhances LDL receptor and PCSK9 expression in vivo and in vitro. J Ethnopharmacol 2011;137:817– 827. DOI: 10.1016/j.jep.2011.06.041.
Riwanto I, Budijitno S, Dharmana E, Handojo D, Prasetyo SA, Eko A, et al. Effect of Phaleria macrocarpa supplementation on apoptosis and tumor growth of C3H mice with breast cancer under treatment with adriamycin-cyclophosphamide. Int Surg 2011;96(2):164-170. DOI: 10.9738/1404.1.
Ali R, B., Atangwho IJ, Kaur N, Abraika OS, Ahmad M, Mahmud R, et al. Bioassay-Guided Antidiabetic Study of Phaleria macrocarpa Fruit Extract. Molecules 2012;17(5):4986-5002. DOI: 10.3390/molecules17054986.
Abood WN, Abdulla MA, Ismail S. Involvement of Inflammatory Mediators in the Gastroprotective Action of Phaleria macrocarpa against Ethanol-Induced Gastric Ulcer. World Applied Sciences Journal 2014;30:344-350.
Tandrasasmita OM, Lee JS , Baek SH , Tjandrawinata RR. Induction of cellular apoptosis in human breastcancer by DLBS1425, a Phaleria macrocarpa compound extract, via downregulation of PI3-kinase/AKT pathway. Cancer Biol Ther 2010;8(10):814-823. DOI: 10.4161/cbt.10.8.13085.
Trusheva B, Trunkova D, Bankova V. Different extraction methods of biologically active components from propolis: a preliminary study. Chem Cent J 2007;1(13):1-4. DOI: 10.1186/1752-153X-1-13.
Clark JD, Gebhart GF, Gonder JC, Keeling ME, Kohn DF. The 1996 guide for the care and use of laboratory animals. ILAR J 1997;38(1):41-48. DOI: 10.1093/ilar.38.1.41.
Morton JJ, Malone MH. Evaluation of vulneray activity by an open wound procedure in rats. Arch Int Pharmacodyn Ther 1972;196(1):117-126.
Suzuki H, Nishizawa T, Tsugawa H, Mogami S, Hibi T. Roles of oxidantive stress in stomach disorders. J Clin Biochem Nutr 2012;50(1):35-39. DOI: 10.3164/jcbn.11-115SR.
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;72(1):248-254. DOI: 10.1016/0003-2697(76)90527-3.
Hinz B, Pittet P, Smith-Clerc J, Chaponnier C, Meister J-J. Myofibroblast development is characterized by specific cell-cell adherens junctions. Mol Bio Cell 2004;15(9):4310-4320. DOI: 10.1091/mbc.E04-05-0386.
Tang T, Yin L, Yang J, Shan G. Emodin, an anthraquinone derivative from Rheum officinale Baill, enhances cutaneous wound healing in rats. Eur J Pharm 2007;567(3):177-185. DOI: 10.1016/j.ejphar.2007.02.033.
Upadhyay A, Chattopadhyay P, Goyary D, Mazumder PM, Veer V. Eleutherine indica L. accelerates in vivo cutaneous wound healing by stimulating Smad-mediated collagen production. J Ethnopharmacol 2013;146(2):490-494. DOI: 10.1016/j.jep.2013.01.012.
Wu X-b, Luo X-q, Gu S-y, Xu J-h. The effects of Polygonum cuspidatum extract on wound healing in rats. J Ethnopharmacol 2012;141(3):934-937. DOI: 10.1016/j.jep.2012.03.040.
Johansen JS, Harris AK, Rychly DJ, Ergul A. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol 2005;4(1):5. DOI: 10.1186/1475-2840-4-5.
Gouthamchandra K, Mahmood R, Manjunatha H. Free radical scavenging, antioxidant enzymes and wound healing activities of leaves extracts from Clerodendrum infortunatum L. Environ Toxicol Pharmacol 2010;30(1):11-18. DOI: 10.1016/j.etap.2010.03.005.
Nevin K, Rajamohan T. Effect of topical application of virgin coconut oil on skin components and antioxidant status during dermal wound healing in young rats. Skin Pharmacol Physiol 2010;23(6):290-297. DOI: 10.1159/000313516.
El-Razek FA, El-Metwally E, Shehab G, Hassan A, Gomaa A. Effects of cactus pear (Opuntia ficus indica) juice on oxidative stress in diabetic cataract rats. Saudi J Health Sci 2012;1(1):23. DOI: 10.4103/2278-0521.94980.
Ali R, B., Atangwho IJ, Kuar N, Mohamed EAH, Mohamed AJ, Asmawi MZ, et al. Hypoglycemic and anti-hyperglycemic study of Phaleria macrocarpa fruits pericarp. J Med Plant Res 2012;6(10):1982-1990.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2015-02-17
Published 2015-05-12









