Soft tissue grafts for dural reconstruction after meningioma surgery
DOI:
https://doi.org/10.17305/bjbms.2019.3949Keywords:
Soft tissue grafts, dura mater, dural reconstruction, brain surgery, cerebrospinal fluid leak, CSF leak, convexity meningiomaAbstract
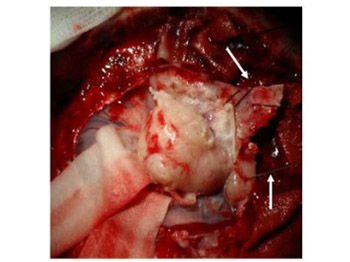
The meninges are involved in various pathologies and are often directly or indirectly severed during surgical procedures, especially the dura mater. This can pose a real challenge for the surgeon, as a proper reconstruction of the meninges is important to prevent complications such as cerebrospinal fluid leak (CSF). A variety of techniques for dural reconstruction have been described, employing natural and artificial materials. A novel technique for dural reconstruction involves soft tissue grafts in the form of fibrous or fibromuscular flaps, which are placed on the dural defects to seal the gaps. These soft tissue grafts represent an appropriate scaffold for cell ingrowth and fibrosis, thus preventing CSF. In this pilot study, we described the application of soft tissue grafts for dural reconstruction in 10 patients who underwent convexity meningioma surgery.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2018-11-26
Published 2019-08-20









