Limb-girdle muscular dystrophy due to GMPPB mutations: A case report and comprehensive literature review
DOI:
https://doi.org/10.17305/bjbms.2019.3992Keywords:
Dystroglycanopathy, limb-girdle muscular dystrophy (LGMD), guanosine diphosphate mannose (GDP-mannose) pyrophosphorylase B gene, GMPPB mutations, heterozygous mutations, high throughput gene panel sequencingAbstract
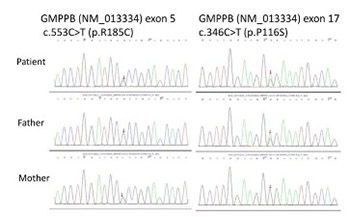
Mutations in the guanosine diphosphate mannose (GDP-mannose) pyrophosphorylase B (GMPPB) gene are rare. To date, 72 cases with GMPPB gene mutations have been reported. Herein, we reported a case of a 29-year-old Chinese male presenting with limb-girdle muscular dystrophy (LGMD) who was found to have two heterozygous GMPPB mutations. The patient had a progressive limb weakness for 19 years. His parents and elder brother were healthy. On examination he had a waddling gait and absent tendon reflexes in all four limbs. Electromyography showed myogenic damage. Muscle magnetic resonance imaging (MRI) showed fatty degeneration in the bilateral medial thigh muscles. High-throughput gene panel sequencing revealed that the patient carried compound heterozygous mutations in the GMPPB gene, c.553C>T (p.R185C, maternal inheritance) and c.346C>T (p.P116S, paternal inheritance). This case provides additional information regarding the phenotypic spectrum of GMPPB mutations in the Chinese population.
Citations
Downloads
Downloads
Additional Files
Published
How to Cite
Accepted 2018-12-19
Published 2020-05-01









