Role of S180L polymorphism in etiology of malaria caused by Plasmodium falciparum in a small group of Pakistani population
DOI:
https://doi.org/10.17305/bjbms.2015.413Keywords:
S180L, polymorphism, malaria, Plasmodium falciparum, MAL/TIRAPAbstract
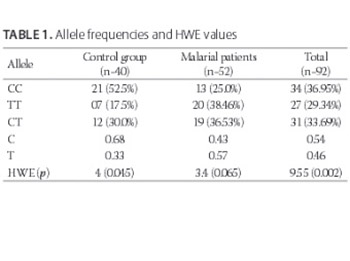
The aim of our study was to investigate the role of S180L polymorphism in modulation of acquisition of malaria caused by Plasmodium falciparum in a small group of Pakistani population. A total of 133 individuals including 60 controls and 73 patients of malaria, caused by Plasmodium falciparum, were genotyped using allele-specific PCR. Ninety-two samples successfully demonstrated the PCR amplification results, while forty-one samples could not be genotyped due to failure in PCR amplification. The allele frequency for S180L polymorphism was deviant from Hardy-Weinberg equilibrium (HWE) of the population under observation. Association was found between the observed polymorphism and the occurrence of malaria caused by Plasmodium falciparum (p = 0.01). Chances of malaria caused by Plasmodium falciparum were low in CC genotype carriers in comparison to other genotypes (Odds ratio: 0.3016; 95% CI: 0.124-0.729). The present findings suggest that S180L polymorphism is important in modulating the probability of acquisition of malaria caused by Plasmodium falciparum in Pakistani population. The CC genotype plays a protective role in local population against this type of malaria.
Citations
Downloads
References
World Health Organization. WHO supports malaria epidemic prevention and control in Pakistan.WHO; 2010, Oct. [cited 2015 July 17]. Available from: www.who.int/hac/crises/pak/releases/12october2010/en/.
Trampuz A, Jereb M, Muzlovic I, Prabhu R M. Clinical review: Severe malaria. Crit Care 2010;7(4):315-323. doi:http://dx.doi.org/10.1186/cc2183.
World Health Organization. WHO Global Malaria Programme. World Malaria Report 2012. Geneva:The WHO Press; 2012 [cited 2015 July 17]. Available from: http://www.who.int/malaria/publications/world_malaria_report_2012/report/en.
Brouqui P, Parola P, Raoult D. Insecticide resistance in mosquitoes and failure of malaria control. Expert Rev Anti Infect Ther 2012;10(12):1379-1381. doi: http://dx.doi.org/10.1586/eri.12.141.
Takida K, Akira S. Toll-like receptors in innate immunity. Int Immunol 2005;17(1):1-14. doi: http://dx.doi.org/10.1093/intimm/dxh186.
Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol 2001;1:134-145. doi: http://dx.doi.org/10.1038/35100529.
Valkov E, Stamp A, Dimaio F, Baker D, Verstak B, Roversi P, et al. Crystal structure of Toll-like receptor adaptor MAL/TIRAP reveals the molecular basis for signal transduction and disease protection. Proc Natl Acad Sci U S A 2011;108(36):14879-14874. doi: http://dx.doi.org/10.1073/pnas.1104780108.
de Mendonçsa VR, Goncalves, MS, Barrel-Netto M. The Host Genetic Diversity in Malaria Infection. J Trop Med 2012;2012:940616. doi: http://dx.doi.org/10.1155/2012/940616.
Kumpf O, Giamarellos-Bourboulis EJ, Koch A, Hamann L, Mouktaroudi M, Djin-Ye O., et al. Influence of genetic variations in TLR4 and TIRAP/Mal on the course of sepsis and pneumonia and cytokine release: an observational study in three cohorts. Crit.Car 2010;14(3): R103. doi: http://dx.doi.org/10.1186/cc9047.
Khor CC, Chapman SJ, Vannberg FO, Dunne A, Murphy C, Ling EY, et al. A Mal functional variant is associated with protection against invasive pneumococcal disease, bacteremia, malaria and tuberculosis. Nat Genet 2007;39:523-528. doi: http://dx.doi.org/10.1038/ng1976.
Zakeri S, Pirahmadi S, Mehrizi AA, and Djadid ND. Genetic variation of TLR-4, TLR-9 and TIRAP genes in Iranian malaria patients. Malar J 2011;10:77. doi: http://dx.doi.org/10.1186/1475-2875-10-77.
Hamann L, Kumpf O, Schuring RP, Alposy E, Bedu-Addo G, Bienzle U, et al. Low frequency of the TIRAP S180L polymorphism in Africa, and its potential role in malaria, sepsis, and leprosy. BMC Med Genet 2009;10:65. doi: http://dx.doi.org/10.1186/1471-2350-10-65.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2015-04-28
Published 2015-08-19









