Predictors of early cardiac changes in patients with type 1 diabetes mellitus: An echocardiography-based study
DOI:
https://doi.org/10.17305/bjbms.2019.4250Keywords:
Type 1 diabetes mellitus, T1DM, body mass index, BMI, left ventricular mass, left atrial volume, diastolic dysfunction, tissue Doppler imagingAbstract
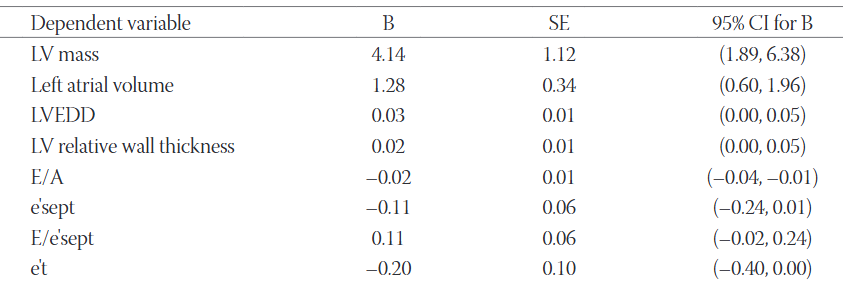
In patients with type 1 diabetes mellitus (T1DM) imaging studies have demonstrated an increased prevalence of left ventricular diastolic dysfunction and increased left ventricular mass (LVM) unrelated to arterial hypertension and ischemic heart disease. The aim of our study was to identify potential predictors of early subclinical changes in cardiac chamber size and function in such patients. Sixty-one middle-aged asymptomatic normotensive patients with T1DM were included in the study. Conventional and tissue Doppler echocardiography was performed and fasting serum levels of glucose, glycated hemoglobin (HbA1c), lipids, and creatinine were measured. We found moderate bivariate correlations of body mass index (BMI) with left atrial volume (r = 0.47, p < 0.01), LVM (r = 0.42, p < 0.01), left ventricular relative wall thickness (r = 0.32, p = 0.01), and all observed parameters of diastolic function of both ventricles. The five-year average value of HbA1c weakly correlated with the Doppler index of left ventricular filling pressure E/e´sept (r = 0.27, p = 0.04). We found no significant association of diabetes duration, five-year trend of HbA1c, serum lipids, and glomerular filtration rate with cardiac structure and function. After adjusting for other parameters, BMI remained significantly associated with left atrial volume, LVM as well as with the transmitral Doppler ratio E/A. In our study, BMI was the only observed parameter significantly associated with subclinical structural and functional cardiac changes in the asymptomatic middle-aged patients with T1DM.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2019 Association of Basic Medical Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2019-06-12
Published 2019-06-18









