Interleukins and inflammatory markers are useful in predicting the severity of acute pancreatitis
DOI:
https://doi.org/10.17305/bjbms.2019.4253Keywords:
Acute pancreatitis, etiology, inflammatory markers, interleukins, scoring systems, Ranson’s criteria, IL6Abstract
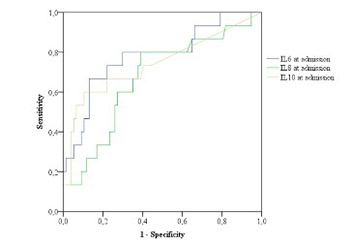
Acute pancreatitis (AP) is a disease with significant morbidity and mortality. The aim of this study was to evaluate the predictive role of inflammatory markers, particularly interleukins (ILs), in the course of AP and to determine the frequency of etiologic factors of AP. We included patients with AP who were treated at our institution from May 1, 2012 to January 31, 2015. Different laboratory parameters, including ILs, and the severity scoring systems Ranson’s criteria and Bedside Index of Severity in Acute Pancreatitis (BISAP) were analyzed. AP was classified into mild and severe, and independent parameters were compared between these groups. The predictive performance of each parameter was evaluated using receiver operating characteristic (ROC) curves and the area under the ROC curve (AUC). A binomial logistic regression was performed to evaluate Ranson’s criteria and IL6, IL8, and IL10 (at admission and after 48 hours) in the course of AP. Overall, 96 patients were treated, 59 (61.5%) males and 37 (38.5%) females, average age 62.5 ± 16.8 years (range 22–91 years). The best predictor for the severity of AP was IL6, measured 48 hours after admission (AUC = 0.84). Other useful predictors of the severity of AP were lactate dehydrogenase (p < 0.001), serum glucose (p < 0.006), and difference in the platelet count (p < 0.001) between admission and after 48 hours (p < 0.001), hemoglobin (p < 0.027) and erythrocytes (p < 0.029). The major causes of AP were gallstones and alcohol consumption. According to our results, IL6 and Ranson score are important predictors of the severity of AP.
Citations
Downloads