Integrin beta 4 (ITGB4) and its tyrosine-1510 phosphorylation promote pancreatic tumorigenesis and regulate the MEK1-ERK1/2 signaling pathway
DOI:
https://doi.org/10.17305/bjbms.2019.4255Keywords:
ITGB4, integrin, MEK1 (T292), invasion, migration, pancreatic cancerAbstract
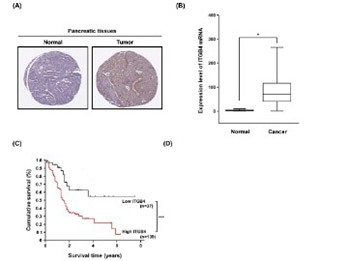
Pancreatic cancer is the fourth leading cause of cancer death, with a 5-year survival rate of only 1–4%. Integrin-mediated cell adhesion is critical for the initiation, progression, and metastasis of cancer. In this study we investigated the role of integrin b4 (ITGB4) and its phosphorylation at tyrosine Y1510 (p-ITGB4-Y1510) in the tumorigenesis of pancreatic cancer. We analyzed the expression of ITGB4 and p-ITGB4-Y1510 in pancreatic cancer tissue and cell lines using immunohistochemistry, Western blot, or semi-quantitative reverse transcription PCR. ITGB4 and p-ITGB4-Y1510 were highly expressed in pancreatic cancer (n = 176) compared with normal pancreatic tissue (n = 171). High p-ITGB4-Y1510 expression correlated with local invasion and distant metastasis of pancreatic cancer, and high ITGB4 was significantly associated with poor survival of patients. Inhibition of ITGB4 by siRNA significantly reduced migration and invasion of PC-1.0 and AsPC-1 cells. Overexpression of the mutant ITGB4-Y1510A (a mutation of tyrosine to alanine at 1510 position) in PC-1.0 and AsPC-1 cells not only blocked the ITGB4 phosphorylation at Y1510 but also suppressed the expression of ITGB4 (p < 0.05 vs. wild-type ITGB4). The transfection of PC-1.0 and AsPC-1 cells with ITGB4-Y1510A significantly decreased the level of p-mitogen-activated protein kinase kinase (MEK)1 (T292) and p-extracellular signal-regulated kinase (ERK)1/2 but did not affect the level of p-MEK1 (T386) and p-MEK2 (T394). Overall, our study showed that ITGB4 and its phosphorylated form promote cell migration and invasion in pancreatic cancer and that p-ITGB4-Y1510 regulates the downstream MEK1-ERK1/2 signaling cascades. Targeting ITGB4 or its phosphorylation at Y1510 may be a novel therapeutic option for pancreatic cancer.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2019-05-29
Published 2020-02-05









