Expression of the anti-Mullerian hormone, kisspeptin 1, and kisspeptin 1 receptor in polycystic ovary syndrome and controlled ovarian stimulation rat models
DOI:
https://doi.org/10.17305/bjbms.2019.4281Keywords:
Anti-Mullerian hormone, kisspeptin, ovarian stimulation, polycystic ovary syndrome, COS, PCOS, KISS-1, KISS1r, GPR54 receptorAbstract
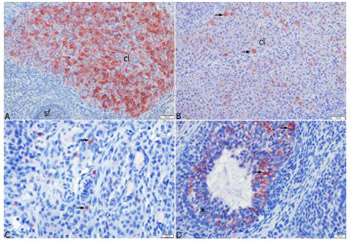
Polycystic ovary syndrome represents a significant cause of female infertility. The aim of this study was to investigate the expression of anti-Mullerian hormone (AMH), kisspeptin 1 (KISS-1), and kisspeptin 1 receptor (KISS1r) in rat models of polycystic ovary syndrome (PCOS) and controlled ovarian stimulation (COS). For this purpose, 28 rats were assigned into four groups. Estrus and Diestrus groups consisted of rats in estrus and diestrus phases, respectively, while COS and PCOS groups consisted of rats with induced COS and PCOS, respectively. The serum AMH, KISS-1, and estradiol levels, and ovarian KISS1r levels were analyzed by enzyme-linked immunosorbent assay. Furthermore, histopathological analysis of the ovary tissue was done and ovarian KISS-1 expression was determined by immunohistochemical assay. The results revealed that ovarian KISS1r levels were higher in the Estrus (1271.43±51.98 pg/mL) and COS (1191.43±85.67 pg/mL) groups, compared to Diestrus and PCOS groups. The highest level of AMH was found in the Estrus group (16.91±2.12 ng/mL). The results indicate that AMH had no effect on the development of COS and PCOS, while KISS-1 was found to affect the development of COS in rats.
Citations
Downloads
Downloads
Additional Files
Published
How to Cite
Accepted 2019-07-03
Published 2020-02-05









