Postoperative pulmonary complications in contemporary cohort of patients with pulmonary hypertension
DOI:
https://doi.org/10.17305/bjbms.2019.4332Keywords:
Pulmonary hypertension, postoperative pulmonary complications, PPCs, post-anesthesia care unit, PACU, respiratory specific eventsAbstract
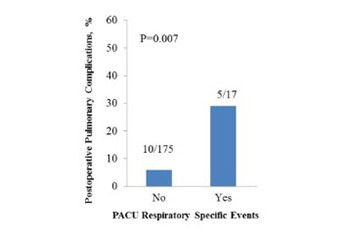
Patients with pulmonary hypertension are at increased risk for postoperative pulmonary complications (PPCs). Herein, we review PPCs in pulmonary hypertension patients undergoing non-cardiac procedures under general anesthesia. The medical records of pulmonary hypertension patients who underwent surgery with general anesthesia between 2010 and 2017 were reviewed for PPCs. In addition we reviewed nursing-documented respiratory depressive episodes in the post-anesthesia care unit to assess the associations between these episodes and later PPCs. There were 20 PPCs among 128 patients who underwent 197 procedures (10.2 per 100 surgeries) [95% CI 6.7–15.2]. Of these, 5 occurred during anesthesia recovery and 15 following anesthesia recovery. Three-quarters of the PPCs occurred within 24 postoperative hours. All the PPCs were severe. The frequency of PPCs was significantly higher in those who experienced respiratory depression during anesthesia recovery vs. in those who did not (5/17, 29% vs. 10/175, 6%; odds ratio 5.15, 95% CI 1.58–16.81, p = 0.007). Increased PPC rates were observed among patients who were current/previous smokers and who routinely use benzodiazepines, and among those undergoing emergent surgery. With treatment, all PPCs resolved. The rate of PPCs in the population of contemporary surgical pulmonary hypertension patients was 10.2%, and three-quarters occurred during first 24 postoperative hours. Patients who had respiratory depression during anesthesia recovery were 5-fold more likely to experience later PPCs.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2019-07-02
Published 2019-11-08









