Effect of SOD2 methylation on mitochondrial DNA4834-bp deletion mutation in marginal cells under oxidative stress
DOI:
https://doi.org/10.17305/bjbms.2019.4353Keywords:
Oxidative stress, superoxide dismutase 2, SOD2, methylation, mtDNA4834 deletion, age-related hearing lossAbstract
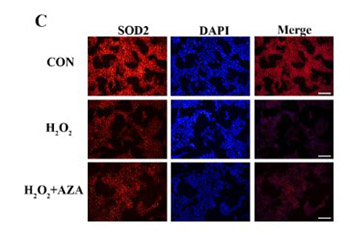
Presbycusis, or age-related hearing loss, is a prevalent disease that severely affects the physical and mental health of the elderly. Oxidative stress and mitochondrial (mt)DNA deletion mutation are considered as major factors in the pathophysiology of age-related hearing loss. The 4977-bp deletion in human mtDNA (common deletion, corresponding to the 4834-bp mtDNA deletion in rats) is suggested to be closely associated with the pathogenesis of age-related hearing loss. Superoxide dismutase 2 (SOD2), an isoform of SOD that is exclusively expressed in the intracellular mitochondrial matrix, plays a crucial role in oxidative resistance against mitochondrial superoxide. Previous research has shown that methylation of the promoter region of the SOD2 gene decreased the expression of SOD2 in marginal cells (MCs) extracted from the inner ear of rats subjected to D-galactose-induced mtDNA4834 deletion. However, the relationship between SOD2 methylation and mtDNA4834 deletion under oxidative stress remains to be elucidated. Herein, an oxidative damage model was established in the extracted MCs using hydrogen peroxide (H2O2), which increased the methylation level of SOD2 and the copy number of mtDNA4834 mutation in MCs. Decreasing the methylation level of SOD2 using 5-azacytidine, a DNA methylation inhibitor, reduced oxidative stress and the copy number of mtDNA4834 mutation and inhibited H2O2-induced apoptosis. The present work demonstrates that decreasing the methylation of SOD2 suppresses the mtDNA4834 deletion in MCs under oxidative stress and provides potential insights to the intervention therapy of aging-related hearing loss.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2019-08-08
Published 2020-02-05









