Tissue-based metabolomics reveals potential biomarkers for cervical carcinoma and HPV infection
DOI:
https://doi.org/10.17305/bjbms.2019.4359Keywords:
Cervical carcinoma, metabolomics, tumor tissue, biomarkersAbstract
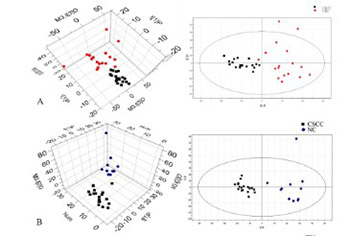
Aberrant metabolic regulation has been observed in human cancers, but the corresponding regulation in human papillomavirus (HPV) infection-associated cervical cancer is not well understood. Here, we explored potential biomarkers for the early prediction of cervical carcinoma based on the metabolic profile of uterine cervical tissue specimens that were positive for HPV16 infection. Fifty-two fresh cervical tissues were collected from women confirmed to have cervical squamous cell carcinoma (SCC; n = 21) or cervical intraepithelial neoplasia (CIN) stages II-III (n = 20). Eleven healthy women constituted the controls (negative controls [NCs]). Real-time polymerase chain reaction (PCR) was performed to detect HPV infection in the tissues. High-resolution magic angle spinning nuclear magnetic resonance was utilized for the analysis of the metabolic profile in the tissues. The expression of rate-limiting enzymes involved in key metabolic pathways was detected by reverse-transcription quantitative PCR. An independent immunohistochemical analysis was performed using 123 cases of paraffin-embedded cervical specimens. A profile of 17 small molecular metabolites that showed differential expression in HPV16-positive cervical SCC or CIN II-III compared with HPV-negative NC group was identified. According to the profile, the levels of α- and β-glucose decreased, those of lactate and low-density lipoproteins increased, and the expression of multiple amino acids was altered. Significantly increased transcript and protein levels of glycogen synthase kinase 3 beta (GSK3β) and glutamate decarboxylase 1 (GAD1) and decreased transcript and protein levels of pyruvate kinase muscle isozyme 2 (PKM2) and carnitine palmitoyltransferase 1A (CPT1A) were observed in the patient group (p < 0.05). HPV infection and cervical carcinogenesis drive metabolic modifications that might be associated with the aberrant regulation of enzymes related to metabolic pathways.
Citations
Downloads
Downloads
Additional Files
Published
How to Cite
Accepted 2019-08-27
Published 2020-02-05









