Inflammatory cells in perivascular adipose tissue and the integrity of the tunica media in atherosclerotic coronary arteries
DOI:
https://doi.org/10.17305/bjbms.2019.4409Keywords:
Perivascular adipose tissue, PVAT, atherosclerosis, coronary arteries, endarterectomy, CAD, coronary artery disease, inflammatory infiltrationAbstract
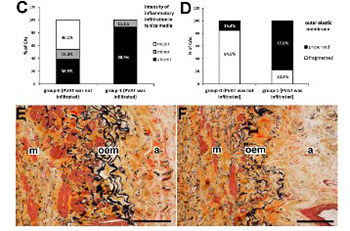
Obstructive coronary artery disease (CAD) is characterized by inflammation within the atherosclerotic coronary arteries. Infiltration of inflammatory cells into muscular media can lead to remodeling and weakening of the arterial wall. We examined the relationship between inflammatory infiltration in perivascular adipose tissue (PVAT), state of the external elastic membrane, and the intensity of inflammatory infiltration in the tunica media of coronary arteries obtained by endarterectomy from symptomatic patients with diffuse CAD. We analyzed endarterectomy sequesters from 22 coronary arteries that contained the intima, media, a part of the adventitia, and PVAT in at least one part of the sequester. The coronary arteries were divided into two groups according to the presence or absence of inflammatory infiltration in PVAT. Staining with hematoxylin-eosin and by the Movat's method showed atherosclerotic changes in the intima and media. Immunohistochemistry (anti-leukocyte common antigen [LCA] antibody) was used for the detection of leukocytes. We found a significant positive correlation between inflammatory infiltration in PVAT and preservation of the external elastic membrane of coronary arteries. Furthermore, we found a significant negative correlation between inflammatory infiltration in PVAT and the intensity of inflammatory infiltration in the media. It seems that the integrity of the external elastic membrane and the proinflammatory properties of PVAT restrain inflammatory cells within PVAT. Both effects may prevent the migration of inflammatory cells into the media and delay the development of CAD.
Citations
Downloads
Downloads
Additional Files
Published
How to Cite
Accepted 2019-09-12
Published 2020-05-01









