Prostate cancer stroma: an important factor in cancer growth and progression
DOI:
https://doi.org/10.17305/bjbms.2015.449Keywords:
Prostate cancer, stroma, myofibroblast, extracellular matrix componentsAbstract
Reactive stromal changes that occur in different human cancers might play a role in local tumor spreading and progression. Studies done on various human cancers have shown activated stromal cell phenotypes, modified extracellular matrix (ECM) composition, and increased microvessel density. Furthermore, they exhibit biological markers consistent with stroma at the site of wound repair. In prostate cancer, stroma is composed of fibroblasts, myofibroblasts, endothelial cells and immune cells. Predominant cells in the tumorous stroma are, however, fibroblasts/ myofibroblasts. They are responsible for the synthesis, deposition and remodeling of the ECM. Epithelial tumorous cells, in interaction with stromal cells and with the help of various molecules of ECM, create a microenvironment suitable for cancer cell proliferation, movement, and differentiation. In this review, we discussed the role of different stromal components in prostate cancer as well as their potential prognostic and therapeutic significance.
Citations
Downloads
References
Hayward SW, Rosen MA, Cunha GR. Stromal-epithelial interactions in the normal and neoplastic prostate. Br J Urol 1997;79(S2):18-26. DOI: 10.1111/j.1464-410X.1997.tb16917.x.
Cunha GR, Ricke W, Thomson A, Marker PC, Risbridger G, Hayward SW, et al. Hormonal, cellular, and molecular regulation of normal and neoplastic prostatic development. J Steroid Biochem Mol Biol 2004;92(4):221–236. DOI: 10.1016/j.jsbmb.2004.10.017.
Berry PA, Maitland NJ, Collins AT. Androgen receptor signalling in prostate: effects of stromal factors on normal and cancer stem cells. Mol Cell Endocrinol 2008;288(1-2):30–37. DOI: 10.1016/j.mce.2008.02.024.
Rowley DR. What might a stromal response mean to prostate cancer progression? Cancer Metastasis Rev 1998;17(4):411-419. DOI: 10.1023/A:1006129420005.
Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res 2002;8(9):2912-2923.
Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119-150. DOI: 10.1146/annurev.pathol.1.110304.100224.
Mueller MM, Fusenig NE. Friends or foes—bipolar effects of the tumour stroma in cancer. Nat Rev Cancer 2004;4(11):839–849. DOI: 10.1038/nrc1477.
Cano P, Godoy A, Escamilla R, Dhir R, Onate SA. Stromal-epithelial cell interactions and androgen receptor-coregulator recruitment is altered in the tissue microenvironment of prostate cancer. Cancer Res 2007;67(2):511-519. DOI: 10.1158/0008-5472.CAN-06-1478.
Tuxhorn JA, Ayala GE, Rowley DR. Reactive stroma in prostate cancer progression. J Urol 2001;166(6):2472-2483. DOI: 10.1016/S0022-5347(05)65620-0.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144(5):646–674. DOI: 10.1016/j.cell.2011.02.013.
Cunha GR, Hayward SW, Wang YZ. Role of stroma in carcinogenesis of the prostate. Differentiation 2002;70(9-10): 473–485. DOI: 10.1046/j.1432-0436.2002.700902.x.
Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer 2003;107(1):1–10. DOI: 10.1002/ijc.11335.
Condon MS. The role of the stromal microenvironment in prostate cancer. Semin Cancer Bio 2005;15(2):132-137. DOI: 10.1016/j.semcancer.2004.08.002.
Celià-Terrassa T, Meca-Cortés O, Mateo F, de Paz AM, Rubio N, Arnal-Estapé A et al. Epithelial–mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. J Clin Invest 2012;122(5):1849–1868. DOI: 10.1172/JCI59218.
Pienta KJ, Abate-Shen C, Agus DB, Attar RM, Chung LW, Greenberg NM, et al. The current state of preclinical prostate cancer animal models. Prostate 2008;68(6):629–639. DOI: 10.1002/pros.20726.
Hensley PJ, Kyprianou N. Modeling prostate cancer in mice: limitations and opportunities. J Androl 2012;33(2):133–44. DOI: 10.2164/jandrol.111.013987.
Zou M, Jiao J, Zou Q, Xu Y, Cheng M, Xu J, et al. Multiple metastases in a novel LNCaP model of human prostate cancer. Oncol Rep 2013;30(2):615–622. DOI: 10.3892/or.2013.2305.
Cunha GR, Tuohimaa P, Visakorpi T. Steroids and prostate cancer. J Steroid Biochem Mol Biol 2004;92(4):219–220. DOI: 10.1016/j.jsbmb.2004.10.001.
Richard C, Kim G, Koikawa Y, Salm SN, Tsujimura A, Wilson EL, et al. Androgens modulate the balance between VEGF and angiopoietin expression in prostate epithelial and smooth muscle cells. Prostate 2002;50(2):83–91. DOI: 10.1002/pros.10035.
Wen S, Chang HC, Tian J, Shang Z, Niu Y, Chang C. Stromal androgen receptor roles in the development of normal prostate, benign prostate hyperplasia, and prostate cancer. Am J Pathol 2015;185:293-301. DOI: 10.1016/j.ajpath.2014.10.012.
Adisetiyo H, Liang M, Liao CP, Jeong JH, Cohen MB, Roy-Burman P, et al. Dependence of castration-resistant prostate cancer (CRPC) stem cells on CRPC-associated fibroblasts. J Cell Physiol 2014;229(9):1170–1176. DOI: 10.1002/jcp.24546.
Rowe RG, Weiss SJ. Navigating ECM barriers at the invasive front: the cancer cell–stroma interface. Annu Rev Cell Dev Biol 2009;25:567–595. DOI: 10.1146/annurev.cellbio.24.110707.175315.
Martin M, Pujuguet P, Martin F. Role of stromal myofibroblasts infiltrating colon cancer in tumor invasion. Pathol Res Pract 1996;192(7):712-717. DOI: 10.1016/S0344-0338(96)80093-8.
Noel A, Foidart JM. The role of stroma in breast carcinoma growth in vivo. J Mammary Gland Biol Neoplasia 1998;3(2):215-225. DOI: 10.1023/A:1018703208453.
Tomas D, Kruslin B. The potential value of (myo)fibroblastic stromal reaction in the diagnosis of prostatic adenocarcinoma. Prostate 2004;61(4):324-331. DOI: 10.1002/pros.20109.
Cirri P, Chiarugi P. Cancer-associated-fibroblasts and tumour cells: a diabolic liaison driving cancer progression. Cancer Metastasis Ver 2012;31(1-2):195–208. DOI: 10.1007/s10555-011-9340-x.
Clark AK, Taubenberger AV, Taylor RA, Niranjan B, Chea ZY, Zotenko E, et al. A bioengineered microenvironment to quantitatively measure the tumorigenic properties of cancer- associated fibroblasts in human prostate cancer. Biomaterials 2013;34(20):4777-4785. DOI: 10.1016/j.biomaterials.2013.03.005.
Wendt MK, Tian M, Schiemann WP. Deconstructing the mechanisms and consequences of TGF-induced EMT during cancer progression. Cell Tissue Res 2012;347(1):85–101. DOI: 10.1007/s00441-011-1199-1.
Gonçalves BF, Campos SG, Costa CF, Scarano WR, Góes RM, Taboga SR. Key participants of the tumor microenvironment of the prostate: an approach of the structural dynamic of cellular elements and extracellular matrix components during epithelial-stromal transition. Acta Histochem 2015;117(1):4-13. DOI: 10.1016/j.acthis.2014.10.009.
Ao M, Brewer BM, Yang L, Franco Coronel OE, Hayward SW, Webb DJ, et al. Stretching fibroblasts remodels fibronectin and alters cancer cell migration. Sci Rep 2015;5:8334. DOI: 10.1038/srep08334.
Tomas D, Spajić B, Milošević M, Demirović A, Marušić Z, Krušlin B. Intensity of stromal changes predicts biochemical recurrence-free survival in prostatic carcinoma. Scand J Urol Nephrol 2010;44(5):284-290. DOI: 10.3109/00365599.2010.485578.
Ayala G, Tuxhorn JA, Wheeler TM, Frolov A, Scardino PT, Ohori M, et al. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin Cancer Res 2003;9(13):4792-4801.
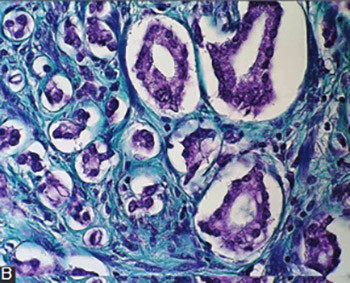
Krušlin B, Tomas D, Rogatsch H, Novosel I, Čupić H, Belicza M et al. Periacinar retraction clefting in the prostatic needle core biopsies: an important diagnostic criterion or a simple artifact? Virchows Arch 2003;443(4):524–527. DOI: 10.1007/s00428-003-0862-7.
Krušlin B, Tomas D, Rogatsch H, Reljić A, Vučić M, Baličević D et al. Correlation of periacinar retraction clefting in needle core biopsies and corresponding prostatectomy specimens. Int J Surg Pathol. 2005;13(1):67-72. DOI: 10.1177/106689690501300109.
Ulamec M, Tomas D, Ensinger C, Čupić H, Belicza M, Mikuz G et al. Periacinar retraction clefting in proliferative prostatic atrophy and prostatic adenocarcinoma. J Clin Pathol 2007;60(10):1098-1101. DOI: 10.1136/jcp.2006.044784.
Fávaro WJ, Hetzl AC, Reis LO, Ferreira U, Billis A, Cagnon VH. Periacinar retraction clefting in nonneoplastic and neoplastic prostatic glands: artifact or molecular involvement. Pathol Oncol Res 2012;18(2):285-292. DOI: 10.1007/s12253-011-9440-5.
Varma M, Lee MW, Tamboli P, Zarbo RJ, Jimenez RE, Salles PG, et al. Morphologic criteria for the diagnosis of prostatic adenocarcinoma in needle biopsy specimens. A study of 250 consecutive cases in a routine surgical pathology practice. Arch Pathol Lab Med 2002;126(5):554-561.
Young RH, Srigley JR, Amin MB, Ulbright TM, Cubilla AL. Tumors of the prostate gland, seminal vesicles, male urethra, and penis, 3th ed. Washington, DC: AFIP; 1998.
Tomas D, Ulamec M, Hudolin T, Bulimbašić S, Belicza M, Krušlin B. Myofibroblastic stromal reaction and expression of tenascin-C and laminin in prostate adenocarcinoma. Prostate Cancer Prostatic Dis. 2006;9(4):414-419. DOI: 10.1038/sj.pcan.4500874.
Irie J, Manucha V, Ioffe OB, Silverberg SG. Artefact as the pathologist's friend: peritumoral retraction in in situ and infiltrating duct carcinoma of the breast. Int J Surg Pathol 2007;15(1):53-59. DOI: 10.1177/1066896906295690.
McKenney JK, Gomez JA, Desai S, Lee MW, Amin MB. Morphologic expressions of urothelial carcinoma in situ: a detailed evaluation of its histologic patterns with emphasis on carcinoma in situ with microinvasion. Am J Surg Pathol. 2001;25(3):356-362. DOI: 10.1097/00000478-200103000-00010.
Tomas D, Spajić B, Milošević M, Demirović A, Marušić Z, Krušlin B. Extensive retraction artefact predicts biochemical recurrence-free survival in prostatic carcinoma. Histopathology. 2011;58(3):447-454. DOI: 10.1111/j.1365-2559.2011.03769.x.
Acs G, Khakpour N, Kiluk J, Lee MC, Laronga C. The presence of extensive retraction clefts in invasive breast carcinomas correlates with lymphatic invasion and nodal metastasis and predicts poor outcome: a prospective validation study of 2742 consecutive cases. Am J Surg Pathol 2015;39(3):325-337. DOI: 10.1097/PAS.0000000000000339.
Acs G, Paragh G, Chuang ST, Laronga C, Zhang PJ. The presence of micropapillary features and retraction artifact in core needle biopsy material predicts lymph node metastasis in breast carcinoma. Am J Surg Pathol 2009;33(2):202-210. DOI: 10.1097/PAS.0b013e318185e171.
Taylor RA, Risbridger GP. Prostatic tumor stroma a key player in cancer progression. Curr Cancer Drug Targets 2008;8(6):490–497. DOI: 10.2174/156800908785699351.
Pupa SM, Menard S, Forti S, Tagliabue E. New insight into the role of extracellular matrix during tumor onset and progression. J Cell Physiol 2002;192(3):259-267. DOI: 10.1002/jcp.10142.
Taboga SR, Vidal BC. Collagen fibers in human prostatic lesions: histochemistry and anisotropies. J Submicrosc Cytol Pathol 2003;35(1):11–16.
LeBleu VS, MacDonald B, Kalluri R. Structure and function of base- ment membranes. Exp Biol Med 2007;232(9):1121–1129. DOI: 10.3181/0703-MR-72.
Patarroyo M, Tryggvason K, Virtanen I. Laminin isoforms in tumor invasion, angiogenesis and metastasis. Semin Cancer Bio. 2002;12(3):197–207.
Colognato H, Yurchenco PD. Form and function: the laminin family of heterotrimers. Dev Dyn 2000;218(2):213–234. DOI: 10.1002/(SICI)1097-0177(200006)218:2<213::AID-DVDY1>3.0.CO;2-R.
Sounni NE, Noel A. Membrane type-matrix metalloproteinase and tumor progression. Biochimie 2005;87(3-4):329-342. DOI: 10.1016/j.biochi.2004.07.012.
Bair EL, Chen ML, McDaniel K, Sekiguchi K, Cress AE, Nagle RB, et al. Membrane type 1 matrix metalloprotease cleaves laminin-10 and promotes prostate cancer cell migration. Neoplasia 2005;7(4):380-389. DOI: 10.1593/neo.04619.
Chiquet-Ehrismann R. Tenascin and other adhesion-modulating proteins in cancer. Semin Cancer Biol 1993;4(5):301-310.
Erickson HP. Tenascin-C, tenascin-R, and tenascin-X: a family of talented proteins in search of their functions. Curr Opin Cell Biol 1993;5(5):869-876. DOI: 10.1016/0955-0674(93)90037-Q.
Shiraishi T, Kato H, Komada S, Imai H, Hirokawa Y, Kusano I, et al. Tenascin expression and postnatal development of the human prostate. Int J Dev Biol 1994;38(2):391-395.
Orend G. Potential oncogenic action of tenascin-C in tumorigenesis. Int J Biochem Cell Biol 2005;37(5):1066-1083. DOI: 10.1016/j.biocel.2004.12.002.
Vollmer G. Biologic and oncologic implications of tenascin-C/hexabrachion proteins. Crit Rev Oncol/Hematol 1997;25(3):187-210. DOI: 10.1016/S1040-8428(97)00004-8.
Van den Brule FA, Waltregny D, Liu FT, Castronovo V. Alteration of the cytoplasmic/nuclear expression pattern of galectin-3 correlates with prostate carcinoma progression. Int J Cancer. 2000;89(4):361-367. DOI: 10.1002/1097-0215(20000720)89:4<361::AID-IJC8>3.0.CO;2-U.
Takenaka Y, Fukumori T, Raz A.Galectin-3 and metastasis. Glycoconj J. 2004;19(7-9):543-549. DOI: 10.1023/B:GLYC.0000014084.01324.15.
Fukumori T, Oka N, Takenaka N, Nangia-Makker P, Elsamman E, Kasai T, et al. Galectin-3 regulates mitochondrial stability and antiapoptotic function in response to anticancer drug in prostate cancer. Cancer Res 2006;66(6):3114-3119. DOI: 10.1158/0008-5472.CAN-05-3750.
Pacis RA, Pilat MJ, Pienta KJ, Wojno K, Raz A, Hogan V, et al. Decreased galectin-3 expression in prostate cancer. Prostate 2000;44(2):118-123. DOI: 10.1002/1097-0045(20000701)44:2<118::AID-PROS4>3.0.CO;2-U.
Wang Y, Nangia-Makker P, Tait L, Balan V, Hogan V, Pienta KJ, et al. Regulation of prostate cancer progression by galectin-3. Am J Pathol 2009;174(4):1515-1523. DOI: 10.2353/ajpath.2009.080816.
Wang M, Berthoud VM, Beyer EC. Connexin43 increases the sensitivity of prostate cancer cells to TNFa-induced apoptosis. J Cell Sci 2007;120(2):320–329. DOI: 10.1242/jcs.03343.
Govindarajan R, Zhao S, Song XH, Guo RJ, Wheelock M, Johnson KR, et al. Impaired trafficking of connexins in androgenindependent human prostate cancer cell lines and its migration by a-catenin. J Biol Chem 2002;277(51):50087–50097. DOI: 10.1074/jbc.M202652200.
Vinken M, Decrock E, De Vuyst E, Ponsaerts R, D'hondt C, Bultynck G, et al. Connexins: sensors and regulators of cell cycling. Biochim Biophys Acta 2011;1815(1):13-25. DOI: 10.1016/j.bbcan.2010.08.004.
Benko G, Spajić B, Demirović A, Stimac G, Kru Sbreve Lin B, Tomas D. Prognostic value of connexin43 expression in patients with clinically localized prostate cancer. Prostate Cancer Prostatic Dis 2011;14(1):90-95. DOI: 10.1038/pcan.2010.51.
Tkachenko E, Rhodes JM, Simons M. Syndecans: new kids on the signaling block. Circ Res 2005;96(5):488–500. DOI: 10.1161/01.RES.0000159708.71142.c8.
Choi S, Kim Y, Park H, Han IO, Chung E, Lee SY, et al. Syndecan- 2 overexpression regulates adhesion and migration through cooperation with integrin alpha2. Biochem Biophys Res Commun 2009;384(2):231–235. DOI: 10.1016/j.bbrc.2009.04.093.
Popović A, Demirović A, Spajić B, Stimac G, Kruslin B, Tomas D. Expression and prognostic role of syndecan-2 in prostate cancer. Prostate Cancer Prostatic Dis 2010;13(1):78-82. DOI: 10.1038/pcan.2009.
Ruiz M, Pettaway M, Stoeltzing O, Ellis L, Bar-Eli M. Activator protein 2 alpha inhibits tumorigenicity and represses vascular endothelial growth factor transcription in prostate cancer cells. Cancer Res 2004;64(2):631–638.DOI: 10.1158/0008-5472.CAN-03-2751.
Chung LWK, Hsieh CL, Law A, Sung SY, Gardner TA, Egawa M, et al. New targets for therapy in prostate cancer: modulation of stromal–epithelial interactions. Urology 2003(5):44–54. DOI: 10.1016/S0090-4295(03)00796-9.
Cooper CR, McLean L, Mucci NR, Poneza P, Pienta KJ. Prostate cancer cell adhesion to quiescent endothelial cells is not mediated by beta-1 integrin subunit. Anticancer Res 2000;20(6B):4159–4162.
Wong YC, Tam NNC. Differentiation of stromal smooth muscle as a factor in prostate carcinogenesis. Differentiation 2002;70(9-10):633-645. DOI: 10.1046/j.1432-0436.2002.700916.x.
Hsieh CL, Gardner TA, Miao L, Balian G, Chung LWK. Cotargeting tumor and stroma in a novel chimeric tumor model involving the growth of both human prostate cancer and bone stromal cells. Cancer Gene Ther 2004;11(2):148–155. DOI: 10.1038/sj.cgt.7700665.
Sakko AJ, Ricciardelli C, Mayne K, Suwiwat S, LeBaron RG, Marshall VR, et al. Modulation of prostate cancer cell attachment to matrix by versican. Cancer Res 2003;63(16):4786-4791.
Desmouliere A, Geinoz, Gabbiani F, Gabbiani G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 1993;122(1):103-111. DOI: 10.1083/jcb.122.1.103.
West AF, O’Donnell M, Charlton RG, Neal DE, Leung HY. Correlation of vascular endothelial growth factor expression with fibroblast growth factor-8 expression and clinico-pathologic parameters in human prostate cancer. Br J Cancer 2001;85(4):576–583. DOI: 10.1054/bjoc.2001.1971.
Djakiew D. Dysregulated expression of growth factors and their receptors in the development of prostate cancer. Prostate 2000;42(2):150-160. DOI: 10.1002/(SICI)1097-0045(20000201)42:2<150::AID-PROS10>3.0.CO;2-H.
Downloads
Additional Files
Published
How to Cite
Accepted 2015-05-04
Published 2015-05-13









