Vancomycin-releasing cross-linked collagen sponges as wound dressings
DOI:
https://doi.org/10.17305/bjbms.2019.4496Keywords:
Wound dressing, sponge, carp, freshwater fish, collagen, vancomycin, infection, crosslinking, MRSAAbstract
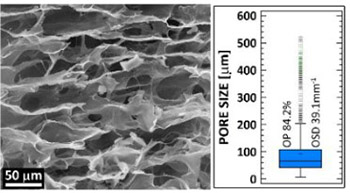
The study presents a novel vancomycin-releasing collagen wound dressing derived from Cyprinus carpio collagen type I cross-linked with carbodiimide which retarded the degradation rate and increased the stability of the sponge. Following lyophilization, the dressings were subjected to gamma sterilization. The structure was evaluated via scanning electron microscopy images, micro-computed tomography, and infrared spectrometry. The structural stability and vancomycin release properties were evaluated in phosphate buffered saline. Microbiological testing and a rat model of a wound infected with methicillin-resistant Staphylococcus aureus (MRSA) were then employed to test the efficacy of the treatment of the infected wound. Following an initial mass loss due to the release of vancomycin, the sponges remained stable. After 7 days of exposure in phosphate buffered saline (37°C), 60% of the material remained with a preserved collagen secondary structure together with a high degree of open porosity (over 80%). The analysis of the release of vancomycin revealed homogeneous distribution of the antibiotic both across and between the sponges. The release of vancomycin was retarded as proved by in vitro testing and further confirmed by the animal model from which measurable concentrations were observed in blood samples 24 hours after the subcutaneous implantation of the sponge, which was more than observed following intraperitoneal administration. The sponge was also highly effective in terms of reducing the number of colony-forming units in biopsies extracted from the infected wounds 4 days following the inoculation of the wounds with the MRSA solution. The presented sponges have ideal properties to serve as wound dressing for prevention of surgical site infection or treatment of already infected wounds.
Citations
Downloads
Downloads
Additional Files
Published
How to Cite
Accepted 2019-11-27
Published 2021-02-01









