Significance of chromogranin A and synaptophysin in pancreatic neuroendocrine tumors
DOI:
https://doi.org/10.17305/bjbms.2020.4632Keywords:
Chromogranin A, immunohistochemistry, multiple endocrine neoplasia 1, pancreatic neuroendocrine tumors, synaptophysinAbstract
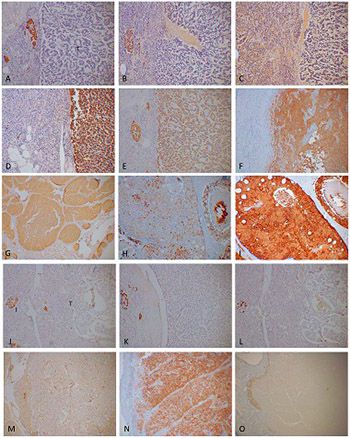
The two most commonly used immunohistochemical markers for neuroendocrine cells and their tumors are chromogranin A (CgA) and synaptophysin (SPY). CgA is a marker for neuroendocrine secretory granules of four pancreatic hormones and gastrin while SPY is a marker for synaptic vesicles in neuroendocrine cells, which release classic neurotransmitters such as acetylcholine and others. CgA is involved in synthesis and secretion of peptide hormones through exocytosis while the function of SPY is elusive. Thirty-five pancreatic neuroendocrine tumors (Pan-NETs) were studied, consisting of 14 insulinomas, 8 gastrinomas, 2 glucagonomas, 6 pancreatic polypeptidomas and 5 non-functioning tumors, and were immunostained for four pancreatic hormones, gastrin, CgA, and SPY. Majority of Pan-NETs were less immunostained for the endocrine hormones and CgA than the normal pancreatic endocrine cells. CgA immunostaining mostly correlates with each hormone staining in non-β-cell tumors, while SPY immunostaining recognizes endocrine cells diffusely in the cytoplasm. CgA immunostaining is less in insulinomas than in non-β-cell tumors, and CgA immunostaining may distinguish CgA-weaker insulinomas from CgA-stronger non-β-cell tumors. CgA immunostaining may be used as an independent marker for biological aggressiveness in non-β-cell Pan-NETs. The serum CgA levels are higher in subjects harboring non-β-cell tumors than those harboring insulinomas, and the serum CgA elevates in parallel to the increasing metastatic tumor mass. Thus, CgA positive immunostaining in Pan-NETs correlates with the elevated serum levels of CgA for diagnosing CgA-positive non-β-cell Pan-NETs and the increasing serum CgA levels indicate increasing metastatic tumor mass.
Citations
Downloads