LAPTM4B promotes the progression of nasopharyngeal cancer
DOI:
https://doi.org/10.17305/bjbms.2020.4738Keywords:
NPC, LAPTM4B, prognosis, proliferation, migration, invasionAbstract
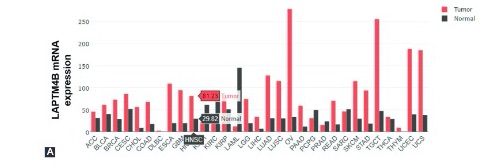
Lysosomal protein transmembrane 4 beta (LAPTM4B) is a protein that contains four transmembrane domains. The impact of LAPTM4B on the malignancy of nasopharyngeal carcinoma (NPC) remains unclear. In the present study, we aimed to investigate the role of LAPTM4B in NPC. NPC tissue samples were used to evaluate the expression of LAPTM4B and its relationship with patient prognosis. Furthermore, we inhibited the expression of LAPTM4B in NPC cell lines and examined the effects of LAPTM4B on NPC cell proliferation, migration, and invasion. We found that LAPTM4B protein was mainly localized in the cytoplasm and intracellular membranes of NPC cells. LAPTM4B protein was upregulated in NPC tissues and cell lines. High LAPTM4B expression was closely related to pathological subtypes and disease stages in NPC patients. NPC patients with high LAPTM4B expression had a worse prognosis. LAPTM4B knockdown inhibited the proliferation, migration, and invasion ability of NPC cells. LAPTM4B plays a cancer-promoting role in the progression of NPC and may be a potential target for NPC therapy.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2020-06-12
Published 2021-06-01









