Clinical management of chronic mercury intoxication secondary to skin lightening products: A proposed algorithm
DOI:
https://doi.org/10.17305/bjbms.2020.4759Keywords:
Mercury, cosmetics, skin-lightening, skin-whitening, bleaching, nephrotic syndrome, neuropsychiatry, dementiaAbstract
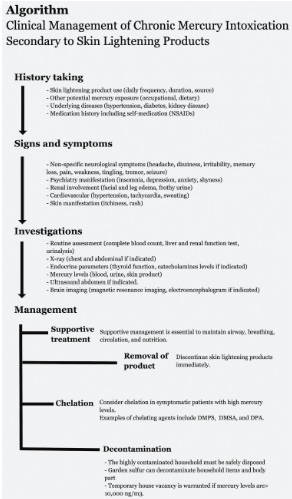
Mercury is a toxic substance that is commonly used in skin lightening products. Various effects on humans have been observed, which affect both users and non-users. Many studies reported delayed diagnosis and treatment, even after weeks of hospitalization. The possible reasons are non-specific clinical manifestation and lack of awareness and knowledge regarding chronic mercury intoxication secondary to skin lightening products. A thorough history of mercury exposure is crucial. Physical assessment and relevant supporting tests are indicated to establish a diagnosis. Blood and urine mercury levels are an essential examination for diagnosis and monitoring of the progress and response to treatment. The primary treatment is the discontinuation of the skin lightening products. Chelation therapy is not mandatory and is usually indicated for symptomatic patients. The prognosis depends on the duration of the product use, concentration of mercury in the skin product, and the severity of clinical presentation.
Citations
Downloads
Downloads
Additional Files
Published
How to Cite
Accepted 2020-05-12
Published 2021-06-01









