Association of polymorphisms in TP53 and the promoter region of IL10 with gastric cancer in a Kazakh population
DOI:
https://doi.org/10.17305/bjbms.2020.4761Keywords:
gastric cancer, polymorphism, IL10, TP53, cytokine, SNPAbstract
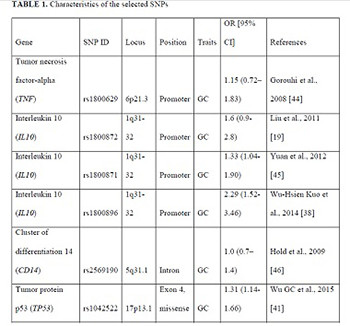
The emerging evidence indicates that single nucleotide polymorphisms (SNPs) of the tumor necrosis factor (TNF), interleukin 10 (IL10), tumor protein p53 (TP53), and cluster of differentiation 14 (CD14) genes may determine individual susceptibility to gastric cancer (GC). We aimed to investigate the associations for polymorphisms of the TNF, IL10, TP53, and CD14 genes in a population of Kazakhs, to identify potential risk or protective associations of the SNPs with GC. A case group of 143 patients hospitalized for GC was enrolled. Controls were 355 volunteers with no history of any cancer and frequency matched with cases by age. Differences in proportions for categorical variables and the assessment of genotypic frequencies conforming to the Hardy–Weinberg equilibrium law were evaluated by the Chi-square test. Associations between genetic polymorphisms and the risk of GC were estimated by regression analysis. For genetic analysis, three genetic models (additive, dominant, and recessive) were used. Four significant associations were found. The SNPs rs1042522 of TP53 and rs1800896 of IL10 were risk factors for GC by the additive model. Two polymorphisms of IL10 were protective of GC, namely, rs1800872 by additive model and rs1800871 by recessive model. No significant associations were observed between the TNF and CD14 polymorphisms and GC. The polymorphisms TP53 rs1042522 and IL10 rs1800896 are associated with GC risk, while the polymorphisms IL10 rs1800872 and rs1800871 are protective of GC in the population of Kazakhs.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2020-07-02
Published 2020-11-02









