Clinical Nocardia species: Identification, clinical characteristics, and antimicrobial susceptibility in Shandong, China
DOI:
https://doi.org/10.17305/bjbms.2020.4764Keywords:
Nocardia, Nocardia infection, nocardiosis, antimicrobial susceptibility, mass spectrometry, identificationAbstract
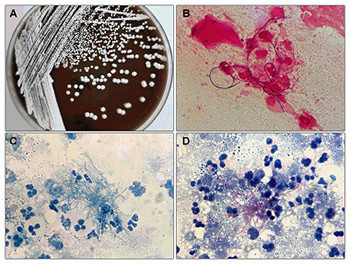
Nocardia is a pathogen responsible for a variety of clinical infections. Here, we aimed to investigate the species distribution, clinical manifestations, and antimicrobial susceptibility of Nocardia species over 3 years in two tertiary general hospitals in China. In this retrospective study, a total of 27 Nocardia species were isolated from 27 individuals between January 2017 and December 2019. Nocardia isolates were identified to species level by mass spectrometry and 16S rRNA PCR sequencing. Clinical data were collected from medical records. Antimicrobial susceptibility was determined by the standard Broth microdilution method. The 27 patients with Nocardia infection included 12 males and 15 females with a mean age of 60.11 years. Among 27 Nocardia isolates, 7 species were identified, with the most common species being Nocardia otitidiscaviarum (40.7%). The antimicrobial susceptibility profiles varied between different Nocardia species. Notably, all Nocardia isolates were linezolid susceptible. The majority of Nocardia isolates were collected from a department of respiratory medicine (55.56%) and sputum specimen (44.44%). Pulmonary region was the most involved body site (70.37%) followed by skin (7.4%) and pleural cavity (7.4%). Most patients with Nocardia infection needed combination antibiotic therapy. Two deaths were reported during the treatment period and 24 patients achieved improvement after antibiotic therapy. The clinical manifestations of Nocardia infection and antimicrobial susceptibility profiles varied with diverse Nocardia species. Thus, the accurate identification of these species is crucial for the diagnosis and the selection of antibiotic treatment.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2020-05-04
Published 2020-11-02









