Dose-dependent effects of adalimumab in neonatal rats with hypoxia/reoxygenation-induced intestinal damage
DOI:
https://doi.org/10.17305/bjbms.2020.4823Keywords:
Adalimumab, hypoxia, intestine, reperfusion, tumor necrosis factor-alphaAbstract
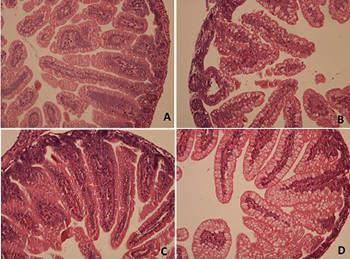
Tumor necrosis factor-alpha (TNF-α) has an important role in hypoxia/reoxygenation (H/R)-induced intestinal damage. It was shown that blocking TNF-α with infliximab has beneficial effects on experimental necrotizing enterocolitis and hypoxic intestinal injury. However, there is no data about the effect of adalimumab on H/R-induced intestinal damage. Therefore, we aimed to determine potential dose-dependent benefits of adalimumab in such damage in neonatal rats. Wistar albino rat pups were assigned to one of the four groups: control group, hypoxia group, low-dose adalimumab (5 mg/kg/day) treated group (LDAT), and high-dose adalimumab (50 mg/kg/day) treated group (HDAT). On the fourth day of the experiment, all rats except for the control group were exposed to H/R followed by euthanasia. Malondialdehyde (MDA), myeloperoxidase (MPO), TNF-α, total antioxidant capacity (TAC), and total oxidant capacity (TOC) were measured in intestinal tissue. TAC and TOC values were used to calculate the oxidative stress index (OSI). Histopathological injury scores (HIS) were also evaluated in the tissue samples. MDA levels were significantly lower in the LDAT and HDAT groups (p < 0.001). TNF-α levels were significantly lower in the LDAT group (p < 0.001). OSI was significantly higher in the H/R group than in the control and LDAT groups (p < 0.001). Mean HIS values in the LDAT group were significantly lower than those in the H/R and HDAT groups (p < 0.001). This experimental study showed that low-dose adalimumab appears to have a beneficial effect on intestinal injury induced with H/R in neonatal rats.
Citations
Downloads
Downloads
Additional Files
Published
How to Cite
Accepted 2020-07-02
Published 2021-02-01









