Postoperative respiratory depression after hysterectomy
DOI:
https://doi.org/10.17305/bjbms.2020.5026Keywords:
Postoperative respiratory depression, postoperative complication, anesthesia recovery period, opioid-induced respiratory depression, hysterectomyAbstract
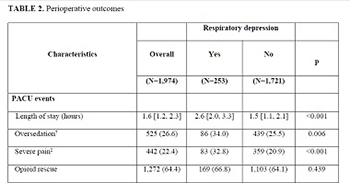
To investigate if sex-specific physiologic characteristics could impact postoperative respiratory depression risks in women, we studied incidence and risk factors associated with postoperative respiratory depression in a gynecologic surgical cohort. Only hysterectomies performed under general anesthesia from 2012 to 2017 were included to minimize interprocedural variability. Respiratory depression was defined as episodes of apnea, hypopnea, hypoxemia, pain-sedation mismatch, unplanned positive airway pressure device application, or naloxone administration in the post-anesthesia care unit. Multivariable logistic regression was used to explore the association with clinical characteristics. From 1,974 hysterectomies, 253 had postoperative respiratory depression, yielding an incidence of 128 (95% confidence interval, 114–144) per 1,000 surgeries. Risk factors associated with respiratory depression were older age (odds ratio 1.22 [95% confidence interval 1.02–1.46] per decade increase, p = 0.03), lower body weight (0.77 [0.62–0.94] per 10 kg/m2, p = 0.01), and higher intraoperative opioid dose (1.05 [1.01–1.09] per 10 mg oral morphine equivalents, p = 0.01); while sugammadex use was associated with a reduced risk (0.48 [0.30–0.75], p = 0.002). Respiratory depression was not associated with increased hospital stay, postoperative complications, or mortality. Postoperative respiratory depression risk in women increased with age, lower weight, and higher intraoperative opioids and decreased with sugammadex use; however, it was not associated with postoperative pulmonary complications.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2020-09-15
Published 2021-06-01









