Cardiotrophin-1: A new predictor of atrial fibrillation relapses after successful cardioversion
DOI:
https://doi.org/10.17305/bjbms.2015.503Keywords:
Atrial fibrillation, cardioversion, cardiotrophin-1Abstract
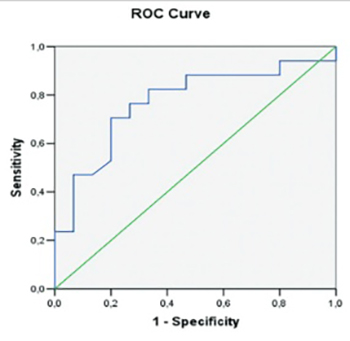
We aimed to investigate whether or not cardiotrophin-1 (CT-1) can be used as a predictor of sinus rhythm constancy in patients with atrial fibrillation (AF) converted to sinus rhythm. Thirty two patients with AF (48-78 years), without any structural heart disease were enrolled for the study. The control group consisted of 32, age and gender matched healthy persons. Measurements of CT-1 were made after transthoracic and transesophageal echocardiography prior to cardioversion (CV). Relapses of AF were investigated by monthly electrocardiograms (ECGs) and ambulatory ECGs at 1st, 3rd, and 6th month. At the end of 6th month, measurements of CT-1 were repeated. At the beginning patients with AF had increased CT-1 levels when compared to controls (0.94 ± 0.32 pg/mL vs. 0.30 ± 0.12 pg/mL, [p < 0.001]). At the end of follow-up of the 32 patients, 17 (53%) had AF relapse. Age, initial duration of AF, left ventricle diameters, ejection fraction, left atrium appendix flow rates were similar among patients with and without AF relapse. However, basal left atrium diameter (4.24 ± 0.14 cm vs. 4.04 ± 0.22 cm, p = 0.005), pulmonary artery pressure (32.82 ± 5 vs. 28.60 ± 6.23 mmHg, p = 0.004) and CT-1 values (1.08 ± 0.37 vs. 0.82 ± 0.16 pg/mL, p = 0.02) were significantly increased in patients with AF relapse. Furthermore, patients with relapsed AF had higher CT-1 levels at 6th month when compared to those in sinus rhythm (1.00 ± 0.40 vs. 0.71 ± 0.23 pg/mL). We conclude that post-CV, AF relapses are more frequent among patients with increased baseline CT-1 levels, and CT-1 may be a potential predictor of AF relapse.
Citations
Downloads
References
Lip GY, Golding DJ, Nazir M, Beevers DG, Child DL, Fletcher RI. A survey of atrial fibrillation in general practice: The West Birmingham Atrial Fibrillation Project. Br J Gen Pract 1997;47(418):285-289.
Kannel WB, Wolf PA, Benjamin EJ, Levy D. Prevalence, incidence, prognosis, and predisposing conditions for atrial fibrillation: Population-based estimates. Am J Cardiol 1998;82(8A):2N-9N.
Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med 1994;154(13):1449-1457.
Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA 1994 16;271(11):840-844. http://www.dx.doi.org/10.1001/jama.271.11.840
http://www.dx.doi.org/10.1001/jama.1994.03510350050036.
Petty GW, Khandheria BK, Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Predictors of cerebrovascular events and death among patients with valvular heart disease: A population-based study. Stroke 2000;31(11):2628-2635. http://www.dx.doi.org/10.1161/01.STR.31.11.2628.
Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res 2002;54(2):230-246. http://www.dx.doi.org/10.1016/S0008-6363(02)00258-4.
Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation 1997;96(4):1180-1184. http://www.dx.doi.org/10.1161/01.CIR.96.4.1180.
Ausma J, Litjens N, Lenders MH, Duimel H, Mast F, Wouters L, et al. Time course of atrial fibrillation-induced cellular structural remodeling in atria of the goat. J Mol Cell Cardiol 2001;33(12):2083-2094. http://www.dx.doi.org/10.1006/jmcc.2001.1472.
Boldt A, Wetzel U, Lauschke J, Weigl J, Gummert J, Hindricks G, et al. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart 2004;90(4):400-405. http://www.dx.doi.org/10.1136/hrt.2003.015347.
Park JH, Pak HN, Choi EJ, Jang JK, Kim SK, Choi DH, et al. The relationship between endocardial voltage and regional volume in electroanatomical remodelled left atria in patients with atrial fibrillation: comparison of three-dimensional computed tomographic images and voltage mapping. J Cardiovasc Electrophysiol 2009;20(12):1349-1356. http://www.dx.doi.org/10.1111/j.1540-8167.2009.01557.x.
Pennica D, King KL, Shaw KJ, Luis E, Rullamas J, Luoh SM, et al. Expression cloning of cardiotrophin 1, a cytokine that induces cardiac myocyte hypertrophy. Proc Natl Acad Sci U S A 1995;92(4):1142-1146. http://www.dx.doi.org/10.1073/pnas.92.4.1142.
Sheng Z, Pennica D, Wood WI, Chien KR. Cardiotrophin-1 displays early expression in the murine heart tube and promotes cardiac myocyte survival. Development 1996;122(2):419-428.
Yoshida K, Taga T, Saito M, Suematsu S, Kumanogoh A, Tanaka T, et al. Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc Natl Acad Sci U S A 1996;93(1):407-411. http://www.dx.doi.org/10.1073/pnas.93.1.407.
Liao Z, Brar BK, Cai Q, Stephanou A, O'Leary RM, Pennica D, et al. Cardiotrophin-1 (CT-1) can protect the adult heart from injury when added both prior to ischaemia and at reperfusion. Cardiovasc Res 2002;53(4):902-910. http://www.dx.doi.org/10.1016/S0008-6363(01)00531-4.
Hausenloy DJ, Yellon DM. Cardioprotective growth factors. Cardiovasc Res 2009;83(2):179-194. http://www.dx.doi.org/10.1093/cvr/cvp062.
Hongya H, Yujie Z, Congya B, Zhe F, Zhenxian Y, Hanying M, et al. Role of cardiotrophin-1 in a canine model of atrial fibrillation and the effect of irbesartan on cardiotrophin-1. Heart 2011;97 Suppl 3:A25. http://www.dx.doi.org/10.1136/heartjnl-2011-300867.71.
Ruiz-Hurtado G, Gómez-Hurtado N, Fernández-Velasco M, Calderón E, Smani T, Ordoñez A, et al. Cardiotrophin-1 induces sarcoplasmic reticulum Ca(2) leak and arrhythmogenesis in adult rat ventricular myocytes. Cardiovasc Res 2012;96(1):81-89. http://www.dx.doi.org/10.1093/cvr/cvs234.
Tsai CT, Lin JL, Lai LP, Lin CS, Huang SK. Membrane translocation of small GTPase Rac1 and activation of STAT1 and STAT3 in pacing-induced sustained atrial fibrillation. Heart Rhythm 2008;5(9):1285-1293. http://www.dx.doi.org/10.1016/j.hrthm.2008.05.012.
Calabrò P, Limongelli G, Riegler L, Maddaloni V, Palmieri R, Golia E, et al. Novel insights into the role of cardiotrophin-1 in cardiovascular diseases. J Mol Cell Cardiol 2009;46(2):142-148. http://www.dx.doi.org/10.1016/j.yjmcc.2008.11.002.
López B, González A, Lasarte JJ, Sarobe P, Borrás F, Díaz A, et al. Is plasma cardiotrophin-1 a marker of hypertensive heart disease? J Hypertens 2005;23(3):625-632. http://www.dx.doi.org/10.1097/01.hjh.0000160221.09468.d3.
Talwar S, Squire IB, Downie PF, Davies JE, Ng LL. Plasma N terminal pro-brain natriuretic peptide and cardiotrophin 1 are raised in unstable angina. Heart 2000;84(4):421-424. http://www.dx.doi.org/10.1136/heart.84.4.421.
Talwar S, Squire IB, O'brien RJ, Downie PF, Davies JE, Ng LL. Plasma cardiotrophin-1 following acute myocardial infarction: relationship with left ventricular systolic dysfunction. Clin Sci (Lond) 2002;102(1):9-14. http://www.dx.doi.org/10.1042/CS20010105.
Tsutamoto T, Asai S, Tanaka T, Sakai H, Nishiyama K, Fujii M, et al. Plasma level of cardiotrophin-1 as a prognostic predictor in patients with chronic heart failure. Eur J Heart Fail 2007;9(10):1032-1037. http://www.dx.doi.org/10.1016/j.ejheart.2007.07.015.
Konii H, Sato K, Kikuchi S, Okiyama H, Watanabe R, Hasegawa A, et al. Stimulatory effects of cardiotrophin 1 on atherosclerosis. Hypertension 2013;62(5):942-950. http://www.dx.doi.org/10.1161/HYPERTENSIONAHA.113.01653.
Mittal S, Ayati S, Stein KM, Schwartzman D, Cavlovich D, Tchou PJ, et al. Transthoracic cardioversion of atrial fibrillation: comparison of rectilinear biphasic versus damped sine wave monophasic shocks. Circulation 2000;101(11):1282-1287. DOI: 10.1161/01.CIR.101.11.1282.
Capucci A, Villani GQ, Aschieri D, Rosi A, Piepoli MF. Oral amiodarone increases the efficacy of direct-current cardioversion in restoration of sinus rhythm in patients with chronic atrial fibrillation. Eur Heart J 2000;21(1):66-73. http://www.dx.doi.org/10.1053/euhj.1999.1734.
Boriani G, Diemberger I, Biffi M, Domenichini G, Martignani C, Valzania C, et al. Electrical cardioversion for persistent atrial fibrillation or atrial flutter in clinical practice: predictors of long-term outcome. Int J Clin Pract 2007;61(5):748-756. http://www.dx.doi.org/10.1111/j.1742-1241.2007.01298.x.
García-Cenador MB, Lopez-Novoa JM, Díez J, García-Criado FJ. Effects and mechanism of organ protection by cardiotrophin-1. Curr Med Chem 2013;20(2):246-256. http://www.dx.doi.org/10.2174/0929867311320020005.
http://www.dx.doi.org/10.2174/092986713804806702.
Khan SQ, Kelly D, Quinn P, Davies JE, Ng LL. Cardiotrophin-1 predicts death or heart failure following acute myocardial infarction. J Card Fail 2006;12(8):635-640. http://www.dx.doi.org/10.1016/j.cardfail.2006.06.470.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2015-06-19
Published 2015-07-23









