Tranexamic acid is associated with decreased transfusion, hospital length of stay, and hospital cost in simultaneous bilateral total knee arthroplasty
DOI:
https://doi.org/10.17305/bjbms.2020.5060Keywords:
Tranaxemic acid, transfusion, total knee arthroplasty, hospital length of stay, healthcare costAbstract
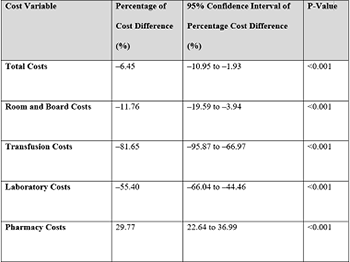
Tranexamic acid (TXA) reduces blood loss and transfusion rates in unilateral total knee arthroplasty (TKA), but there is limited data regarding its efficacy in bilateral TKA. This study reports the impact TXA has on clinical outcomes and hospital cost of care in simultaneous, primary bilateral TKA. The 449 patients were retrospectively reviewed. Primary outcomes included the rates of allogeneic and autologous blood transfusion. Secondary outcomes included hospital length of stay (HLOS), post-hospital discharge disposition, 30-day thromboembolic events (TEE), and mean hospital cost of care. Total direct medical costs were obtained from an institutional research database and adjusted to nationally representative unit costs in 2013 inflation-adjusted dollars. Our study revealed that in patients undergoing simultaneous bilateral TKA, TXA use was associated with reduced allogeneic (OR 0.181, 95% CI 0.090-0.366, p < 0.001) and combined allogeneic and autologous transfusion rates (OR 0.451, 95% CI 0.235-0.865, p = 0.017). TXA was associated with a HLOS reduction of 0.9 days (β-coefficient −0.582, 95% CI −1.008-−0.156, p = 0.008), an increased likelihood of hospital discharge over skilled nursing facility (SNF) (OR 2.25, 95% CI 1.117-4.531, p = 0.023) and reduced total hospital cost of care by 6.45% (p < 0.001), room and board costs by 11.76% (p < 0.001), and transfusion costs by 81.65% (p < 0.001). In conclusion, TXA use in bilateral TKA is associated with lower blood transfusion rates, reduced hospital length of stay, reduced cost of hospital care and skilled nursing facility avoidance.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2020-10-21
Published 2021-08-01









