Development and validation of prognostic nomogram for lung cancer patients below the age of 45 years
DOI:
https://doi.org/10.17305/bjbms.2020.5079Keywords:
Early-onset lung cancer, prognostic nomogram, overall survival, cancer-specific survival, SEERAbstract
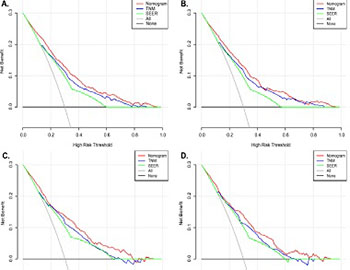
This study aimed to establish a nomogram for the prognostic prediction of patients with early-onset lung cancer (EOLC) in both overall survival (OS) and cancer-specific survival (CSS). EOLC patients diagnosed between 2004 and 2015 were retrieved from the Surveillance, Epidemiology, and End Results (SEER) database and further divided into training and validation sets randomly. The prognostic nomogram for predicting 3-, 5- and 10-years OS and CSS was established based on the relative clinical variables determined by the multivariate Cox analysis results. Furthermore, the predictive performance of nomogram was assessed by concordance index (C-index), calibration curve, receiver operating characteristic (ROC) curve and decision curve analysis (DCA) curve. A total of 1,822 EOLC patients were selected and randomized into a training cohort (1,275, 70%) and a validation cohort (547, 30%). The nomograms were established based on the statistical results of Cox analysis. In training set, the C-indexes for OS and CSS prediction were 0.797 (95% confidence interval [CI]: 0.773-0.818) and 0.794 (95%CI:0.771-0.816). Significant agreement in the calibration curves was noticed in the nomogram models. The results of ROC and DCA indicated nomograms possessed better predict performance compared with TNM-stage and SEER-stage. And the area under the curve (AUC) of the nomogram for OS and CSS prediction in ROC analysis were 0.766 (95%CI:0.745-0.787) and 0.782 (95%CI:0.760-0.804) respectively. The prognostic nomogram provided an accurate prediction of 3-, 5-, and 10-year OS and CSS of EOLC patients which contributed clinicians to optimize individualized treatment plans.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2020-10-11
Published 2021-06-01









