Microsurgical resection of giant T11/T12 conus cauda equina schwannoma
DOI:
https://doi.org/10.17305/bjbms.2020.5153Keywords:
schwannoma, conus, cauda equina, microsurgery, gross total resectionAbstract
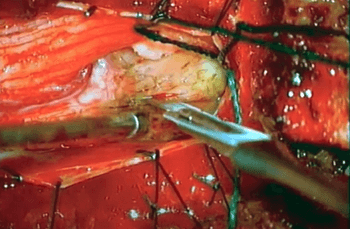
In this video, we highlight the anatomy involved with microsurgical resection of a giant T11/T12 conus cauda equina schwannoma. Spinal schwannoma remains the third most common intradural spinal tumor. Tumors undergoing gross total resection usually do not recur. To our knowledge, this is the first video case report of giant cauda equina schwannoma resection. A 55-year-old female presented with paraparesis and urinary retention. Lumbar spine MRI revealed a contrast-enhancing intradural extramedullary tumor at the T11/T12 level. Surgery was performed in the prone position with intraoperative neurophysiology monitoring (somatosensory and motor evoked potentials—SSEPs and MEPs). T11/T12 laminectomies were performed. After opening the dura and arachnoid, the tumor was found covered with cauda equina nerve roots. We delineated the inferior pole of the tumor, followed by opening of the capsule and debulking the tumor. Subsequently, the cranial pole was dissected from the corresponding cauda equina nerve roots. Finally, the tumor nerve origin was identified and divided after nerve stimulation confirmed the tumor arose from a sensory nerve root. The tumor was removed; histological analysis revealed a schwannoma (WHO Grade I). Postoperative MRI revealed complete resection. The patient fully recovered her neurological function. This case highlights the importance of careful microsurgical technique and gross total resection of the tumor in the view of favorable postoperative neurological recovery of the patient. Intraoperative use of ultrasound is helpful to delineate preoperatively tumor extension and confirm postoperative tumor resection.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2020-09-29
Published 2021-08-01









