Accelerated atherosclerosis in premenopausal women with rheumatoid arthritis – 15-year follow-up
DOI:
https://doi.org/10.17305/bjbms.2020.5176Keywords:
Rheumatoid arthritis, atherosclerosis, cardiovascular disease, metalloproteasesAbstract
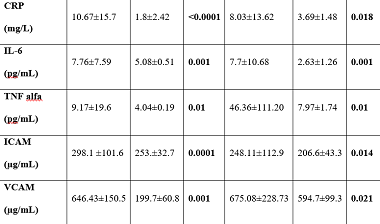
Rheumatoid arthritis (RA) is a chronic inflammatory disease associated with increased mortality and morbidity due to the higher cardiovascular risk in these patients. Traditional risk factors are not the only answer for the accelerated atherosclerosis. In a long-term prospective study, we investigated the relationship between asymptomatic atherosclerosis and traditional risk factors and inflammatory markers in patients with RA and matched healthy controls. We studied the laboratory test results, the concentrations of inflammatory mediators, matrix metalloproteases (MMP), and inflammation markers in a total of 70 (60 at follow-up) premenopausal healthy women with RA and 40 (34 at follow-up) matched controls. We used the B-mode ultrasound imaging of carotid arteries for the detection of asymptomatic atherosclerosis. Correlation with different factors was evaluated. Statistically significant higher values of inflammatory markers such as selective adhesion molecules ICAM and VCAM, interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-alpha), and MMP-3 in the patients group were found in the follow-up study. More plaques were found in the patients group (42.4% vs. 12.9%; p=0.005), as compared with the controls group. The patients had also higher values of cIMT (p=0.001). Using bivariate regression analysis only VCAM was found as a prognostic factor for plaque occurrence (r= 0. 341, p=0.016), but not for cIMT (r= -0.130, p=0.327) in premenopausal female patients with RA after the follow-up. Therefore, asymptomatic atherosclerosis is accelerated in premenopausal women with RA. The results of our follow-up study showed the association between inflammation and accelerated atherosclerosis. Furthermore, VCAM was found to have a statistically significant correlation with plaque occurrence in these patients.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2020-11-17
Published 2021-08-01









