Clinical significance of miR-129-5p in patients with neonatal sepsis and its regulatory role in the LPS-induced inflammatory response
DOI:
https://doi.org/10.17305/bjbms.2020.5184Keywords:
Neonatal sepsis, miR-129-5p, diagnosis, inflammatory responseAbstract
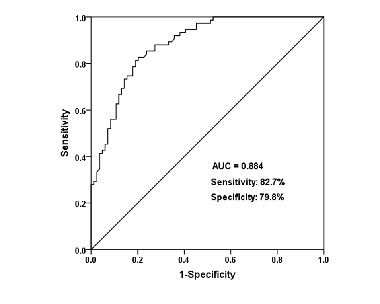
Neonatal sepsis (NS) occurs in neonates within 28 days, especially preterm infants. The dysregulation of miRNAs is widely detected in NS. The study investigated the expression changes and clinical significance of miR-129-5p in NS patients and further explored the regulatory role of miR-129-5p in the LPS-induced inflammatory response in monocytes. A total of 75 neonates with NS and 84 neonates without NS were recruited. qRT-PCR was used for the measurement of miR-129-5p expression. The receiver operating characteristic (ROC) curve was constructed for diagnostic value analysis. ELISA was used to detect the concentration of inflammatory cytokines. Monocytes were isolated from the blood of neonates to investigate the role of miR-129-5p in the LPS-induced inflammatory response in vitro. miR-129-5p was low expressed in the serum of NS cases compared with controls. Serum miR-129-5p had a diagnostic value for NS with a sensitivity of 82.7% and specificity of 79.8%. There was close association for serum miR-129-5p with TNF-α (r = -0.652, p < 0.001) and IL-8 (r = -0.700, p < 0.001) levels in NS patients. Overexpression of miR-129-5p reversed the increasing trend of TNF-α and IL-8 induced by LPS, whereas miR-129-5p downregulation aggravated the increase of TNF-α and IL-8 induced by LPS in monocytes. MiR-129-5p was downregulated in the serum of NS patients, and it might be a promising biomarker for disease diagnosis. Overexpression of miR-129-5p alleviated the inflammatory response of NS.
Citations
Downloads
Downloads
Additional Files
Published
How to Cite
Accepted 2020-12-23
Published 2021-04-01









